Article Text
Abstract
Objective To assess cost-effectiveness of newborn screening (NBS) for spinal muscular atrophy (SMA) and early treatment with nusinersen or onasemnogene abeparvovec (gene therapy), compared with nusinersen without SMA screening.
Methods Informed by an Australian state-wide SMA NBS programme, a decision analytical model nested with Markov models was constructed to evaluate costs and quality-adjusted life-years (QALYs) from a societal perspective with sensitivity analyses.
Results By treating one presymptomatic SMA infant with nusinersen or gene therapy, an additional 9.93 QALYs were gained over 60 years compared with late treatment in clinically diagnosed SMA. The societal cost was $9.8 million for early nusinersen treatment, $4.4 million for early gene therapy and $4.8 million for late nusinersen treatment. Compared with late nusinersen treatment, early gene therapy would be dominant, gaining 9.93 QALYs while saving $360 000; whereas early nusinersen treatment would result in a discounted incremental cost-effectiveness ratio (ICER) of $507 000/QALY.
At a population level, compared with no screening and late treatment with nusinersen, NBS and early gene therapy resulted in 0.00085 QALY gained over 60 years and saving $24 per infant screened (85 QALYs gained and $2.4 million saving per 100 000 infants screened). More than three quarters of simulated ICERs by probability sensitivity analyses showed NBS and gene therapy would be dominant or less than $50 000/QALY, compared with no screening and late nusinersen treatment.
Conclusion NBS coupled with gene therapy improves the quality and length of life for infants with SMA and would be considered value-for-money from an Australian clinical and policy context.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article. This paper reports the modelling cost-effectiveness with published study results and statistics from the pilot study. Data sources of the model parameters are presented in Table 1.
Statistics from Altmetric.com
Introduction
Spinal muscular atrophy (SMA) with an incidence of 1 in 10 000 live births is an autosomal recessive neuromuscular disease caused by biallelic mutations in the survival motor neuron 1 (SMN1) gene, resulting in degeneration of motor neurons in the spinal cord, progressive muscle weakness and atrophy.1–3 The best-known determinant of severity of SMA is the number of copies of SMN2, a back-up gene to SMN1, with copy numbers that vary between individuals.2 The benefits of early diagnosis and presymptomatic initiation of treatment are now established in infantile and childhood SMA following the clinical implementation of the disease-modifying therapies, nusinersen and onasemnogene abeparvovec (gene therapy).4 To expedite treatment initiation and optimise outcomes, newborn screening (NBS) for SMA has been introduced or is being considered in several countries.
Previous economic evaluations have shown nusinersen was not cost-effective when administered in symptomatic people with SMA whereas gene therapy was cost-effectiveness compared with supportive care and/or nusinersen on the basis of preliminary efficacy data.5–10 Cost-effectiveness analysis of disease-modifying therapies in the era of universal screening is limited.9 An economic evaluation of NBS for SMA in the era of advanced therapeutics is important in guiding decision-making regarding their economic value and serves as an exemplar for their translation into clinical practice to guide sustainable health policy in rare neurogenetic diseases.11
Methods
This economic evaluation aims to assess, from the Australian societal perspective, both short-term and long-term cost-effectiveness of early identification of SMA by NBS with treatment by disease-modifying therapies, that is, nusinersen or gene therapy, compared with (1) nusinersen treatment without screening (primary comparator); (2) a historical cohort without screening and managed by supportive care in the era prior to nusinersen (secondary comparator).
Decision analytic model for NBS
We used the Treeage software Pro 2020 software (TreeAge Software, Williamstown, Massachusetts, USA) to develop a decision analytic model of NBS versus no NBS nested with Markov models representing treatment options. Depending on the screening pathway (screening vs no screening) and the treatment option (nusinersen, gene therapy or supportive care), a series of Markov cohort simulations were conducted reflecting the health outcomes and costs for each infant in the population according to four treatment strategies (figure 1): (1) NBS to enable early identification and treatment initiation with nusinersen (Markov Clone 1); (2) NBS to enable early identification and treatment initiation of with gene therapy (Markov Clone 2); (3) symptomatic diagnosis by clinical referral and treated with nusinersen from the time of diagnosis (‘primary comparator’ the current practice in Australia, Markov Clone 3) and (4) symptomatic diagnosis by clinical referral and managed by supportive care (‘secondary comparator’).
Decision analytic models for SMA NBS. NBS, newborn screening; SMA, spinal muscular atrophy.
Parameters for the decision analytic model were derived from the New South Wales/Australian Capital Territory NBS pilot programme’s laboratory data. Health outcomes were reported as quality-adjusted life-years (QALYs). All costs and QALYs were discounted at 3% per annum to the reference year 2018.12 An infant with a confirmed SMA diagnosis detected by NBS (true positives) would either initiate nusinersen treatment (Markov Clone 1) or receive gene therapy after diagnosis (Markov Clone 2). False negatives later presenting with symptoms would start the disease-modifying nusinersen treatment in a postsymptomatic stage and enter the primary comparator Markov model (Markov Clone 3).
Markov models for SMA disease progression with and without disease-modifying therapies
Markov cohort simulations are stochastic processes that allow individuals to transition from one health state to another. Our Markov models focused on motor milestone achievement during the disease course and were guided by published literature regarding SMA natural history and clinical trials in various age groups and SMA genotypes and phenotypes. We defined the time length of a Markov cycle as 6 months, based on clinical observations of motor milestone development in SMA. In the Markov models, 11 health states were specified to capture SMA disease evolution (figure 2). Every infant diagnosed with SMA started with the non-sitter state and advanced to acquire a set of motor milestones using the WHO criteria, that is, sitting without support, standing with assistance, walking with assistance, standing and walking alone.13 Transition probabilities were weighted based on Australian SMA epidemiological data by genotype (2 copies 69% and 3 copies 31%) for NBS-detected SMA and by phenotype (SMA1 58%, SMA2 29%, SMA3 13%) for symptomatic diagnosis by clinical referral.1 11 Loss of motor milestones could occur, depending on the SMA phenotype and treatment modality received. If loss of milestones occurred, it was assumed one-step backward transition occurred and the individual would stay at the regressed state till death, except loss of sitting that could regress further to permanent ventilation for SMA2.14 Only non-sitters could transition to the state requiring permanent ventilation and subjects could die in any health state.15 Model parameters, values and their sources of valuation are presented in table 1.
Diagraph presentation of the health states modelled in the Markov model.
Model parameters and expected values with ranges
Data sources to inform the Markov models transition probabilities
NBS and treatment with nusinersen or gene therapy
In the NBS intervention cohort, two disease-modifying treatment options, that is, nusinersen or gene therapy, were modelled to account for costs and outcomes for the screen-detected patients with SMA. Treatment effectiveness was determined by the NURTURE study that reported median ages of first achievement of milestone to estimate the transition probabilities between health states.4 No deaths or loss of motor milestones were reported in the NURTURE study at median follow-up of 2.9 years, and population background mortality was used. Gene therapy is currently under investigation for newborns with SMA.16 Therefore, we applied the same treatment efficacy for nusinersen treatment in presymptomatic SMA.
Nusinersen treatment without screening
Two randomised, sham-controlled phase 3 efficacy trials of nusinersen for infantile-onset SMA, and later-onset SMA, the ENDEAR and CHERISH studies, respectively, were used to estimate the transition probabilities of motor milestones for the primary comparator Markov model.17 18 The SHINE study, an ongoing open-label extension study of the ENDEAR study further reporting nusinersen treatment for infantile-onset SMA provided data in milestone achievements for a median 3.4 years follow-up.19
Supportive care only without screening
Observational studies that have been conducted to characterise the natural history of SMA in patients prior to the advent of disease-modifying therapies were used for model parameters, including number of patients and ages at motor milestone acquisition and loss, and the weighted transition probabilities. For children with SMA type 1 managed with supportive care we used the study by Finkel et al.20 We assumed that once non-invasive ventilation was commenced for at least 16 hours/day, it was permanent. For patients with SMA type 2 and 3 we referred to the prospective longitudinal study by Chabanon et al for acquisition and loss of psychomotor development milestones.21
Quality of life
Quality of life (QoL) utility values were assigned to each health state to generate QALYs. Utility values were sourced from an Australian study on the prenusinersen economic and health-related QoL burden that included a detailed cost analysis of 40 infants and children with SMA.22 We reanalysed QoL of the Australian data by motor status and supplemented by QoL estimates in a US community survey study.23 Utility values for the five first-acquired motor function health states are presented in table 1. It was also assumed that individuals with loss of motor function had lower QoL and a disutility percentage was applied.
Costs
Costs from the societal perspective included costs of screening (including true and false positives), diagnosis, disease-modifying therapies, direct medical care, informal care and parents’ loss of productivity to care for SMA children.22 24–28
The screening and diagnosis costs were collected from our pilot NBS programme. Treatment costs of nusinersen were based on the NURTURE study treatment regimen, consisting of four loading doses in the first 2 months followed by a maintenance dose every 4 months.4 The reimbursement cost $A110 000 (~US$75 810) for nusinersen 12 mg/5 mL injection by the Australia Pharmaceutical Benefit Scheme was used for one injection.27 For each nusinersen injection and gene therapy episode, a same-day admission was required to undertake the procedures and postinjection observation. Cost weights of the Australian Refined Diagnosis Related Grooup code together with published price by the Australian Independent Hospital Price Authority were used to estimate the cost of treatment episode (including sedation, lumbar puncture and all procedures).26 As the market price of gene therapy is unknown in Australia, a lifetime one-off pharmaceutical cost of US$1.54 million was assigned in the base case, based on an overseas comparable price, with realistic ranges incorporating benchmark full price in the USA for sensitivity analyses.24 29 Postgene therapy follow-up was undertaken with 10 specialist consultations in the first year and then biannually. Annual direct medical costs and indirect care costs were obtained from the Australian SMA cost study.22 All prices were adjusted to 2018 US$ Purchasing Power Parity values.30
Cost-effectiveness analysis
Incremental costs and QALYs were compared with calculate the incremental cost-effectiveness ratios (ICERs). Two sets of cost-effectiveness analysis were performed. First, four SMA treatment strategies (Markov models) for one infant diagnosed with SMA were modelled over 5 and 60 years. Incremental costs and QALYs were calculated for each pair comparison between SMA treatment strategies and reported as incremental cost per patient diagnosed with SMA.
Second, the cost-effectiveness of NBS and early disease-modifying therapies (decision analytical model plus four nested Markov treatment strategies) estimated the costs and QALYs for one infant born in the population, and compared with no NBS and clinical diagnosis with late nusinersen treatment or supportive care.
Sensitivity analysis
One-way sensitivity analysis was performed to identify the most influential parameters on the results and a tornado diagram was generated to visually illustrate the effect of these parameters on ICERs. Probabilistic sensitivity analysis was conducted using the relevant model parameters with distributions obtained from the literature (table 1). Monte Carlo simulation was performed for 1000 iterations to generate cost-effectiveness planes with 95% CIs. Scenario analysis was conducted to test the assumptions made regarding the costs of nusinersen and gene therapy.
Results
Cost-effectiveness of SMA treatment strategies
Total and incremental costs and QALYs for one infant diagnosed with SMA by four treatment strategies evaluated by Markov cohort simulations over 5 and 60 years are presented in table 2. Figure 3 illustrates total costs and QALYs for these four SMA treatment strategies over 60 years.
Costs and QALYs per spinal muscular atrophy case treated by four therapy strategies over 5 and 60 years, and ICERs for pairwise comparisons from the societal perspective, discounted 3% per annum
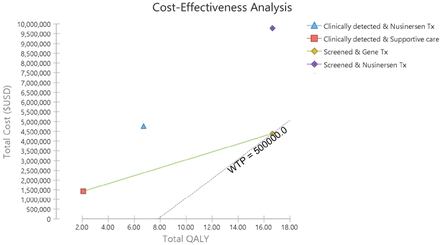
Cost-effectiveness plane for four spinal muscular atrophy treatment strategies by Markov cohort simulation over 60 years from the societal perspective, discounted 3%per annum. QALY, quality-adjusted life-year; WTP, willingness to pay
In the long-term pair-wise comparison of the treatment strategies for one diagnosed SMA, early treatment with nusinersen gained additional 9.93 QALYs at additional cost $5.03 million over 60 years, yielding an ICER $507 000/QALY, compared with late symptomatic nusinersen treatment. In contrast, comparing to late initiation of nusinersen in clinically diagnosed SMA, gene therapy in screen-detected SMA would be dominant achieving 9.93 QALYs gain and saving $0.36 million over a 60-year projection. Compared with supportive care only, gene therapy in screen detected SMA resulted in an ICER $202 000/QALY (14.61 QALYs gain at $2.95 million).
Cost-effectiveness of NBS for SMA including treatment strategies
Total and incremental costs and QALYs, and ICERs for one infant screened versus not screening in the population are presented in table 3. Compared with no screening and late nusinersen treatment, NBS with presymptomatic nusinersen treatment would gain additional 0.00007 QALYs at additional $33 per infant screened (7 QALYs at $3.3 million in a 100 000 cohort) resulting in ICER $494 000/QALY in the short-term. NBS with presymptomatic gene therapy would achieve the same QALY gains with additional $48 per infant screened (7 QALYs gains at $4.8 million in a 100 000 cohort), resulting $714 000/QALY in the base case.
Costs and QALYs per newborn by four screening and treatment strategies over 5 and 60 years, and ICERs for pairwise comparisons from societal perspective, discounted 3% per annum
In the long-term, screening every newborn and treating diagnosed SMA with nusinersen for 60 years would cost $867 per infant and result in 0.00162 QALYs ($86.7 million and 162 QALYs in 100 000 infants). Compared with no screening and late nusinersen treatment, NBS would gain 0.00085 QALY at an additional cost $436 per infant resulting in an ICER $513 000/QALY. Screening every newborn in the population and treating diagnosed SMA infants with gene therapy would achieve the same health gain but at a much lower total cost ($407 vs $867 per infant). Compared with late nusinersen treatment without screening, NBS and early gene therapy would be dominant in the base case with 95% CI of ICER from dominant to $239 000/QALY.
Sensitivity analysis
Results of the probability sensitivity analysis for NBS are presented in figure 4. Compared with symptomatic nusinersen treatment, NBS with gene therapy would be either dominant or cost-effective using the commonly accepted willingness-to-pay threshold $50 000/QALY. Seventy-seven per cent simulated ICERs (green dots in figure 4) either fall in the dominant quadrant (QALY gains and cost saving) or below the $50 000/QALY threshold.
Costs-effectiveness plane for NBS and gene therapy compared with late nusinersen treatment without screening over 60 years from the societal perspective, discounted 3% per annum. NBS, newborn screening; QALY, quality-adjusted life-year; WTP, willingness to pay
One-way sensitivity analysis for the cost-effectiveness of NBS with gene therapy compared with nusinersen without NBS indicated the most significant variable was nusinersen maintenance treatment cost. If current nusinersen price was discount by 26% to reduce half year maintenance cost at US$88 239, ICER for gene therapy compared with late nusinersen treatment will reach the willingness-to-pay threshold $50 000/QALY (figure 5). Other influential factors impacting on ICERs included SMA incidence (higher SMA incidence resulting in lower ICERs), and cost of gene therapy. While various SMA milestone progression probabilities were tested, variations of these parameters had little impact on the total QALYs.
Tornado diagram of one-way sensitivity analysis on ICER for NBS and gene therapy compared with late nusinersen treatment without screening from the societal perspective, discounted 3% per annum. ICER, incremental cost-effectiveness ratio; NBS, newborn screening; SMA, spinal muscular atrophy; WTP, willingness to pay; EV, expected value.
Informed by one-way sensitivity analysis results, a scenario analysis on the costs of disease-modifying treatments was undertaken to show the impact of price changes on ICERs. Most noticeably, a change of the gene therapy cost from the base case value would change the conclusion of cost-effectiveness for this new therapeutic option. At the US$1.9 million price, total discounted costs for NBS and early gene therapy over 60 years would be $4.75 million and lost the dominant status above $1.9 million. If the gene therapy costs increased to US$2.1 million, total discounted treatment costs would be $4.95 million over 60 years; and compared with nusinersen without NBS, the ICER for NBS with gene therapy would change from dominant to $21 000/QALY, which is still considered cost-effective in Australian healthcare setting.
Discussion
This paper presents cost-effectiveness analyses for early identification of SMA by NBS versus SMA diagnosis without NBS coupled with treatments including gene therapy and nusinersen. Although this evaluation uses modelling to project short-term and long-term costs and outcomes, it is based on real-world data incidence and test performance from the ongoing pilot study in Australia, and QoL and cost data collected in a previous Australian survey of 40 SMA children and caregivers.22 Therefore, the results represent the epidemiological and clinical decision-making in an Australian setting and prudence in relating these to other healthcare settings is essential. In addition, our model corresponds with nusinersen and onasmenogene abeparvovec clinical trials, which have not included presymptomatic newborns with >3 SMN2 copies, and is consistent with regulatory approvals for onasemnogene abeparvovec in several regions (eg, European Medicines Agency, Brazil and Australia) specifying SMA with 1–3 copies of SMN2, such that our model is likely to be of broad interest.4 16
Our methodology is comparable in modelling to other cost-effectiveness studies using a decision-analytical model with Markov simulations with assumptions made for extrapolation of lifetime consequences.5–9 15 31 32 Our model further improves the analysis with better QoL utility estimates by applying direct measures from individuals impacted by SMA or parent/caregiver proxy measures in Australia and the USA,22 23 compared with previous economic evaluations5–7 using mapping from PedsQL to EQ5D or deriving case vignettes of SMA type I, II and III with clinical experts rating by EQ5D.33–35
Introduction of NBS and treatment with nusinersen is unlikely to be cost-effective for a therapy under rare disease cost-effective thresholds.36 Our findings are in line with the results of the cost-effectiveness analyses for SMA treated by nusinersen reported in the literature.5–9 15 31 32 Our analysis indicates that gene therapy is the favourable treatment modality compared with nusinersen accounting for lifetime direct medical and indirect informal care costs in the context of NBS within the Australian healthcare system. Furthermore, at the US benchmark price $2.1 million for gene therapy, NBS coupled with gene therapy is considered cost-effective with an ICER $21 000/QALY in the long-term based on a generally accepted willingness-to-pay thresholds of $50 000/QALY. Although wide 95% CIs, our conclusion of NBS with gene therapy being a preferred choice is compelling as more than three quarters of simulated ICERs are less than $50 000/QALY (figure 4). Large variations in the costs and outcomes reflect our broad consideration in costs and treatment effects.
Cost-effectiveness has previously been assessed in gene therapy by comparing to best supportive care or nusinersen.6 9 10 15 Two studies incorporated NBS in the analysis8 9 while some cost-effectiveness analysis studies only modelled infantile-onset SMA.6–8 Our findings are comparable to the conference abstract of Chen et al reporting an ICER of $187 650/QALY for NBS with gene therapy, compared with nusinersen treatment in clinically identified SMA. The preliminary findings of Chen et al also concluded that NBS with presymptomatic nusinersen treatment is dominated by NBS with gene therapy.9 Different to previous cost-effectiveness analyses,5 7 8 our paper presents both the short-term ICERs to provide results with confidence in disease-modifying treatment effect for SMA supported by available clinical evidence and the long-term ICERs to account for life-time costs and benefits from NBS.
Our study has limitations in particular the need to model over a life-long timeframe with available short-term clinical data. However, we validated our models 5-year results against the literature with similar durations of follow-up and found good calibrations. Thus, results from the 5-year modelling are of high confidence to support NBS with gene therapy, given the treatment effect of gene therapy in other conditions (eg, Parkinson’s disease) has been observed for 8–10 years.37 It is not currently feasible to redose gene therapy and our model assumed one dose is durable over a lifetime. It is also inevitable to assume constant transition probabilities between health states over the entire Markov process, implying treatment benefits continued beyond available observed outcomes. The caveat of such assumptions needs careful considerations in using the life-time modelled results. Furthermore, in modelling the long-term costs, we made two assumptions. First, we applied constant lifelong nusinersen treatment for both with and without NBS as when and how nusinersen maintenance injections can be altered are yet unknown. Second, we may have over-estimated the ongoing direct medical and indirect care costs with nusinersen and gene therapy because the annual costs from the Australian costing study were based on a prenusinersen SMA cohort which included several SMA cases in advanced disease progression with supportive care.22 This may also underestimate infantile SMA non-sitters as ventilation was not part of routine supportive care prenusinersen and supportive care practices are changing.1 The cost-effectiveness of NBS and SMA treatment for >3SMN2 is a pertinent and evolving model of care, necessitating further research and complicated by broad genotype-phenotype correlations, from SMA type 1 to adult SMA, in retrospective studies.38
In terms of QALY estimates, unlike the assumptions made by others,7 15 we are conservative in modelling QALY for NBS by assuming no QoL difference between symptomatic and presymptomatic SMA cases. Use of cross-sectional QoL estimates from older patients with SMA22 23 in treated infants may not be ideal but these estimates are the best available source before prospective data are collected. Our model is also in favour of nusinersen because the burden of treatment for children and families associated with repeated intrathecal nusinersen injections is not accounted for. Future analysis is warranted to illuminate the real impact by disease-modifying therapies on QoL when prospective QoL data are collected from children treated with nusinersen and gene therapy for extended periods. The QoL for individuals identified by NBS and initiating early treatment, with some achieving early childhood motor milestones in line with developmental expectations, is not yet established and may further impact the cost-effectiveness, as utility values and requirements for supportive care for these potential ‘SMA free’ phenotypes may differ to the model comparator of an ambulant SMA type 3 individual.
Although untreated SMA was the leading genetic cause of infant death, it is a rare condition such that usual cost-effectiveness thresholds applied to mainstream medical interventions may not be applicable. The Institute for Clinical and Economic Review adapts a modified approach for ‘potential major advance for a serious ultra-rare condition’ and recommends a broader willingness-to-pay thresholds from $50 000 to $500 000 per QALY.36 The challenges of a specific methodological reference case for the management of ultra-rare and health-catastrophic diseases have also been recognised.39 40 Nevertheless, affordability has not been addressed and should be included in future analysis. Importantly, treatment selection is not limited to costs, with clinical and laboratory characteristics (eg, AAV9 Ab titres, liver function) and family preferences regarding relative efficacy, route and frequency of treatment administration and potential side effects important in therapeutic decision-making.
Conclusion
Early identification of SMA through NBS and treatment with gene therapy is cost-effective compared with either screen-detected or late clinical diagnosis SMA treated with nusinersen. NBS coupled with gene therapy improves the quality and length of life of infants with SMA and is value for money in the Australian context.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article. This paper reports the modelling cost-effectiveness with published study results and statistics from the pilot study. Data sources of the model parameters are presented in Table 1.
Ethics statements
Patient consent for publication
Ethics approval
This study has been approved by The Sydney Children’s Hospitals Network Human Research Ethics Committee (reference number LNR/18/SCHN/307). Parents of the children participating in the NBS pilot evaluation study provided written consents.
Acknowledgments
We are grateful for the funding support from Luminesce Alliance and assistance from the newborn screening pilot study. The NSW Pilot NBS study was funded by Luminesce Alliance, a not-for-profit cooperative joint venture across the Sydney Children’s Hospital Network, Children’s Medical Research Institute and Children’s Cancer Institute, established with the support of the NSW Government. Luminesce Alliance is also affiliated with UNSW Sydney and The University of Sydney.
References
Footnotes
Twitter @SophyShih, @imichellefarrar
Contributors STFS: study design, model construction, data analysis and interpretation, preparation and critical review of the manuscript. MAF: study conceptualisation, study design, data interpretation, manuscript preparation and critical review of the manuscript. VW: data collection, critical review of the manuscript. GC: study conceptualisation, study design, data interpretation, manuscript preparation and critical review of the manuscript.
Funding The NSW Pilot NBS study (no award/grant number) was funded by Luminesce Alliance, a not-for-profit cooperative joint venture across the Sydney Children’s Hospital Network, Children’s Medical Research Institute and Children’s Cancer Institute, established with the support of the NSW Government. Luminesce Alliance is also affiliated with UNSW Sydney and The University of Sydney.
Disclaimer These funding bodies had no role in the design of the study, data collection, data analysis, manuscript design, preparation of the manuscript or decision to publish. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Competing interests MAF has received compensation as a member of the scientific advisory board for Biogen, Roche and AveXis. This study received funding from Luminesce Alliance, a not-for-profit cooperative joint venture across the Sydney Children’s Hospital Network, Children’s Medical Research Institute and Children’s Cancer Institute, established with the support of the NSW Government.
Provenance and peer review Not commissioned; externally peer reviewed.