Article Text
Abstract
Guillain-Barré syndrome (GBS) is an acute polyradiculoneuropathy with a highly variable clinical course and outcome. Intravenous immunoglobulin (IVIg) and plasma exchange are proven effective treatments, but the efficacy has been demonstrated mainly on motor improvement in adults with a typical and severe form of GBS. In clinical practice, treatment dilemmas may occur in patients with a relatively mild presentation, variant forms of GBS, or when the onset of weakness was more than 2 weeks ago. Other therapeutic dilemmas may arise in patients who do not improve or even progress after initial treatment. We provide an overview of the current literature about therapeutic options in these situations, and additionally give our personal view that may serve as a basis for therapeutic decision-making.
- GUILLAIN-BARRE SYNDROME
Statistics from Altmetric.com
Introduction
Guillain-Barré syndrome (GBS) is a rapidly progressive and potentially life-threatening polyradiculoneuropathy that requires early diagnosis, monitoring and treatment.1 ,2 Plasma exchange (PE, usually 200–250 mL/kg in five sessions) and intravenous immunoglobulin (IVIg, 0.4 g/kg for 5 days) are proven effective treatments for GBS.3 ,4 IVIg may be considered first choice treatment because it is relatively easy to administer, widely available and has less side effects.3–5 Despite the proven effectiveness of these treatments in GBS, the care of patients in clinical practice is often complex. First, outcome in many patients is still poor: 2–10% may die, 20% are still unable to walk after 6 months and many patients suffer from residual symptoms, including pain and severe fatigue.1 ,3 ,4 ,6–8 Second, the patients in whom the therapeutic effects have been demonstrated frequently represent a selected proportion of the patients (symptoms <2 weeks and who are walking with aid, bed bound or in need of artificial ventilation (GBS disability grade ≥3, table 1)).
Guillain-Barré syndrome (GBS) disability scale
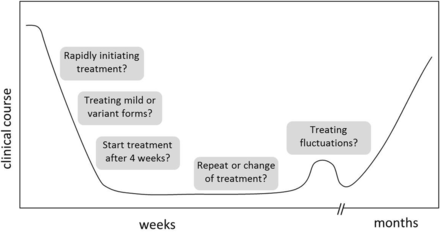
Third, the efficacy of PE and IVIg has primarily been demonstrated related to improvement on the GBS disability scale 4 weeks after the start of treatment. However, this scale focuses on walking and does not take into account other consequences of GBS that are important in daily life, such as arm function, facial weakness, sensory deficits, pain and fatigue. Finally, as a consequence of this, the Cochrane reviews about treatment of GBS are restricted to the specific inclusion and outcome criteria of the trials being focused on the GBS disability scale.3 ,4 ,11–14 In clinical practice, clinicians are facing various situations that are not covered by the existing therapeutic studies and the other literature (figure 1).
Overview treatment dilemmas.
In this review, we will address two main issues that may result in dilemmas in the treatment of patients with GBS:
Start of (standard) treatment
Therapeutic time window
Mild form of GBS
Clinical variants and electrophysiological subtypes of GBS
Children
Change or repeat of treatment
Insufficient clinical response
Add on treatment
Other treatments than PE or IVIg
Treatment-related fluctuations (TRFs)
We give a summary of the current evidence of treatment in these specific clinical situations. Furthermore, we provide a personal view for each dilemma, in order to support clinicians in their decision-making, as long as evidence from clinical trials is lacking. The level of evidence of the treatment effect ranges from 1 to 4 (table 2).
Start of (standard) treatment
Therapeutic time window
Time is nerve?
All randomised controlled trials (RCTs) with IVIg and PE in GBS were conducted in the acute phase of disease, within 2 (in case of IVIg) to 4 weeks (in case of PE) after the onset of weakness. One may assume that treatment is most effective when started as soon as possible in order to prevent further nerve damage, similar to the concept ‘Time is brain’ in ischaemic cerebrovascular accidents. Some support for this hypothesis comes from the PE trials, where PE in patients randomised within 7 days after the onset of symptoms had a more pronounced effect (on time to improve one clinical grade, median time to walk without assistance), than in patients randomised between 8 and 28 days after onset.3 ,17 ,18 Furthermore, IVIg has pleiotropic immune modulatory effects that may inhibit Fc-mediated activation of macrophages, prevent binding of antibodies to neural targets and prevent complement activation which would otherwise lead to further nerve damage.4 ,19 ,20 These effects of IVIg and the potential ongoing nerve injury, in the absence of results of properly controlled trials, may implicate that treatment should be initiated as soon as possible.
Current personal view: Based on limited evidence we recommend to start treatment as soon as possible in patients who walk with aid, are bedbound or ventilated (level of evidence: 3). In patients who are still able to walk unaided but show rapid progression of symptoms one likely should aim to prevent further nerve damage and not wait for further clinical deterioration (level of evidence: 4).
How long after the onset of weakness can treatment still be effective?
The progressive phase in the vast majority of patients with GBS takes <4 weeks, and most patients will present within a few days to weeks after the onset of symptoms.21 However, about 3% of patients may progress during a period of 4–8 weeks that in part may be due to an ongoing immune-mediated injury of the nerves (subacute idiopathic demyelinating polyneuropathy, SIDP).22 For these cases, no evidence is available regarding treatment effect of IVIg or PE.
Presentation 4 weeks after the onset of symptoms can be a demonstration of a relatively mild disease course with a good natural prognosis which does not necessitate treatment. When there is still progression after 4 weeks, especially in patients who are not that severely disabled and who show clear signs of demyelination on nerve conduction studies, acute-onset chronic inflammatory demyelinating polyneuropathy (A-CIDP) should be considered. Especially when progression persists after 8 weeks, chronic inflammatory demyelinating polyneuropathy (CIDP) should be considered and then (re-) treatment with IVIg or even a switch to corticosteroids is indicated.23
Current personal view: There is no information available on the effect of treatment in patients with GBS presenting 4 weeks or later after the onset of weakness. Subacute GBS or A-CIDP should be considered in patients who present after 4 weeks of onset. We suggest to start IVIg when there is clear clinical progression or a ‘wait and see’ policy in case of relatively mild and stable disease (level of evidence: 4).
‘Mildly affected’ patients
Although there is no consensus about the definition of mild GBS, one may consider a patient who is still able to walk unaided to be mildly affected, although it can imply that the patient has other severe neurological deficits. In this paper, like in some other publications, we use the term mild GBS when the patient is still able to walk without help (GBS disability score 1 or 2).27 Previous studies indicate that about one-third of patients have a mild form of GBS, although the actual proportion may be under-reported due to selection bias.24 Some studies have indicated that the clinical course in these patients may not be as mild as expected. Up to 38% of patients with a mild form of GBS-reported problems in hand function and running after 6 months follow-up despite the fact that 22% of them received treatment.25
Most RCTs were conducted in patients with a severe form of GBS, defined as walking with aid or worse (table 1). The primary end point in these trials was usually based on the proportion of patients regaining the capacity to walk unaided or improvement by at least one grade on the GBS disability scale. In part because of these endpoints, mildly affected patients were usually not included in the RCTs, which limits the evidence whether treatment will be effective in this subgroup of patients.
The Cochrane reviews on PE and IVIg provide no direct advice for the treatment of mild GBS.3 ,4 The therapeutic effect of IVIg has not been evaluated in adult patients with mild GBS. However, in a small group of children with mild GBS, a shorter time to improvement and a lower GBS disability grade at 4 weeks were observed in the IVIg group.26
One RCT investigated the effect of PE on time to onset of motor recovery in patients being able to stand unaided, or walk 5 m with or without assistance.27 In this study, it was shown that treatment with two PE sessions significantly shortened the time to onset of motor recovery (4 days) than supportive care (8 days) and shortened the time to hospital discharge (13 vs 18 days).27 Long-term outcome (defined as full muscle strength recovery after 1 year) was not significantly different, but this outcome measurement may lack specificity to demonstrate a difference. Moreover, spontaneous full recovery is possible due to the mild course of the disease, and it would possibly be more informative to investigate whether treatment hastens full recovery in the context of cost-effectiveness and risk–benefit analysis.
Current personal view: Patients with mild GBS may have long-term functional impairment, but only a beneficial effect of treatment with PE has been demonstrated (level of evidence: 2). This effect has not been demonstrated for IVIg in adult patients. Based on the effect of PE in mild cases and of IVIg in severe cases, IVIg likely may be effective in mild GBS too. We propose that treatment (either PE or IVIg) should be considered especially in mildly affected patients who develop additional features such as autonomic dysfunction, bulbar or facial weakness (level of evidence: 4). New treatment trials preferentially should study the effect of treatment not only restricted to severely affected patients with GBS.
Clinical variants and electrophysiological subtypes of GBS
Miller Fisher syndrome (MFS)
MFS, characterised by ophthalmoplegia, ataxia and areflexia, is considered to be a variant form of GBS because of the common underlying pathogenesis and the presence of overlap forms with GBS.28 Patients with typical MFS (ie, without limb weakness) in general have a benign natural course with complete recovery in 60–100% of the patients after 6 months.14 ,29 ,30 Two retrospective studies (total n=142) found no difference in time to complete recovery in patients treated with IVIg or PE versus supportive care, but IVIg slightly hastened the time to onset of amelioration of symptoms.30 ,31
According to the Cochrane review, there is currently not enough evidence that immunotherapy could hasten recovery of MFS and that patients suffering from typical MFS are likely to improve completely with a conservative approach.14
However, 25–50% of patients presenting with MFS will develop limb weakness (MFS-GBS overlap syndrome) and 40% of patients will develop additional bulbar weakness and swallowing disorders that may require intubation.28 ,32 ,33 There currently are no prognostic models available to predict which patients are prone to progress to MFS-GBS overlap syndrome. According to the Cochrane review, results of therapeutic trials in GBS may be extrapolated to patients with a MFS-GBS overlap syndrome because it is part of the GBS spectrum.14
Current personal view: Evidence from retrospective studies indicates that typical MFS might require supportive care only because of the relatively benign natural course (level of evidence: 3). In patients with additional limb weakness, swallowing disorders, facial weakness or respiratory failure treatment with IVIg or PE should be considered (level of evidence: 4).
Bickerstaff's brainstem encephalitis (BBE)
BBE is considered to be a rare variant within the GBS spectrum.34 Patients with BBE usually have ophthalmoplegia, ataxia and sometimes limb weakness, in addition they show symptoms of brainstem involvement including alterations in consciousness or long tract signs.35 No RCT has been conducted in BBE, and only case reports and series have been published describing the clinical course after various forms of treatment. The largest study was a retrospective study in 62 cases of BBE, which reported different combinations of treatment regimens (PE, IVIg, steroids, combinations of these and supportive care).36 Six months after the onset of symptoms, two-third of all patients had completely recovered, with the highest recovery in the IVIg group. Residual symptoms in the other patients were limb weakness, cognitive changes, diplopia, gait disturbance, dysaesthesia and dysphagia. Five per cent (3 patients) of patients died during a 6 month follow-up period. Other smaller series have reported full recovery of neurological symptoms in 67–100% of the patients after 6 months.14
Current personal view: Although the effect of treatment has not properly been studied in BBE, the clinical severity of BBE in the acute phase and overlap with GBS suggests that treatment with IVIg or PE in the acute phase is justifiable (level of evidence: 4).
Other clinical variants of GBS
Other variants within the GBS spectrum are the pure motor, pharyngeal-cervical-brachial (PCB), pure ataxic, pure sensory and paraparetic GBS. No RCT has been performed specifically in any of these variants.
One post-hoc subgroup analysis reported that significantly more patients with pure motor GBS regained the capacity to walk unaided after treatment with IVIg compared with PE (87% vs 45%, p=0.02).37 Three other retrospective studies showed evidence that patients with anti-GM1 antibodies (associated with pure motor GBS) might do better after IVIg compared with PE.38–42
Current personal view: Based on the results of four retrospective studies with small numbers of patients, we consider to recommend IVIg over PE in patients with pure motor GBS (level of evidence: 3). Patients with PCB, ataxic and sensory GBS might never become eligible for treatment when only the GBS disability scale is taken into account and therefore treatment should be initiated when symptoms are seriously disabling or rapidly progressing (level of evidence: 4).
Electrophysiological subtypes of GBS
Based on nerve conduction studies, GBS can be classified into acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor (sensory) axonal neuropathy (AMAN, AMSAN). The proportion of patients with axonal GBS varies between geographical areas, with a higher frequency in Asian and South-American countries.1 Most therapeutic studies were conducted in Western countries where the frequency of axonal GBS is relatively low (<10%). Patients with AMAN or AMSAN were included in these trials but only one study performed a post-hoc analysis in this subgroup, which showed no difference in outcome of these patients treated with either IVIg or PE, although patient numbers were small (total n=32).43
Current personal view: No RCT has been performed exclusively in patients with axonal forms of GBS, until such studies have demonstrated otherwise, we recommend to treat these patients similarly as the patients with a demyelinating form of GBS (level of evidence: 4).
Children with GBS
GBS may occur at all ages, although the incidence of GBS in children is lower than in adults. Three prospective randomised trials have investigated the effect of IVIg versus supportive care in children and one investigated the effect of IVIg versus PE in ventilated children (table 3).
Overview of RCTs in children
The first three studies showed that IVIg had a significant effect on shortening the time to improvement and total recovery than dexamethasone or supportive care.4 ,26 ,44 ,46 It was also found that in 51 severely affected children, there was no difference in effectiveness when IVIg was administered over 2 or 5 days (total 2 g/kg), although there were more relapses (TRFs) in the group with a short treatment regimen.26 The effect of PE has not been investigated extensively in large randomised trials in children. One prospective randomised trial in 41 ventilated children found that PE slightly but significantly shortened the duration of mechanical ventilation compared with IVIg-treated children, but there was no significant effect on hospital stay or the proportion of children able to walk unaided at 4 weeks.45 Important to bear in mind, is that PE in children can have more adverse events and complications than in adults because of citrate toxicity, higher relative vascular volume shifts and the need for safe vascular access.47
Current personal view: There currently is no indication to treat children with GBS differently than adults. IVIg seems to be effective in children with GBS (level of evidence: 2) and is preferred over PE because it is easier to administer and possibly better tolerated in small children (level of evidence: 3).
Change or repeat of treatment
Insufficient clinical response after initial treatment
Patients with GBS may show no signs of clinical recovery after initial treatment and may even further deteriorate. Previous trials have shown that about 40–50% of patients treated with either PE or IVIg show no improvement on the GBS disability scale at 4 weeks (table 4).17 ,18 ,48 ,49 At present it is only possible to evaluate the effect of treatment on a clinical basis. Whether a patient would benefit from a second course or a change to another treatment cannot be determined yet.
Overview of PE or IVIg RCTs with outcome measures based on GBS disability scale
Switch to another therapy
Some neurologists may switch to the other treatment after either IVIg or PE as initial treatment if there is no clinical response. The rationale is that these treatments probably have different immunomodulatory effects that may influence the treatment efficacy in individual patients. One randomised trial compared the efficacy of PE, IVIg, and PE followed immediately by IVIg in 379 severely affected patients, but did not find significant differences between the three treatment modalities in any of the outcome measures.10 Thus IVIg after PE was not significantly better than IVIg or PE alone. However, all patients receiving the combination switched to IVIg regardless of recovery after PE. No trial has been conducted to show whether patients who truly do not respond to one of these two treatments, may respond after switching to the other treatment.
Whether PE after IVIg should be considered remains unclear. One small retrospective study in 46 patients reported that treatment with IVIg followed by PE was not better than IVIg alone. On the contrary, the patients who received both treatments had a worse GBS disability grade at discharge and were longer hospitalised.55 The researchers conclude that this could reflect a more severe disease course in patients receiving two treatments, but it could also suggest that PE washes out IVIg, thus preventing the therapeutic effects of IVIg.
Repeat treatment
Another option for patients who continue to deteriorate after initial treatment is to repeat the same regimen of treatment, being either PE or IVIg. Most studies on PE have investigated the effect of five exchanges. One trial showed that six PEs were not superior over four in already ventilated patients but the sixth course was given as part of the study protocol and not because of lack of improvement.27
A second course of IVIg may be beneficial in patients who rapidly metabolise the administered IgG. Previous studies showed that a low-serum IgG increase 2 weeks after treatment is associated with more a severe disease course and poor outcome in comparison with patients who have a high IgG increase after treatment.56 Four severely affected patients with GBS who did not show recovery after a first course of IVIg started to improve after a second course of IVIg.57 However, this study was not controlled and for these patients it was not possible to determine whether the second course contributed to clinical recovery. A double-blind placebo RCT evaluating the effect of a second course of IVIg (administered shortly after the first IVIg course) in patients with GBS with a poor prognosis is currently being conducted in the Netherlands, the Second IVIg Dose in GBS trial (SID-GBS). The results of this study are awaited in 2018.
Current personal view: At present there is no evidence that outcome is improved by repeating treatment (either IVIg or PE) or switch to another type of treatment (level of evidence: 2). PE after IVIg should probably be avoided (level of evidence: 4).
Add-on treatment to IVIg
Various trials have shown that treatment with corticosteroids alone does not improve recovery in GBS and some studies even suggest that oral corticosteroids may delay recovery.12 One large RCT indicated that intravenous methylprednisolone (500 mg/day for 5 days) when added to IVIg has a small effect at 4 weeks after a post-hoc correction for known prognostic factors, but there was no improvement of long-term outcome.54
Studies in patients and animal models have established the crucial role of complement activation in the pathogenesis of GBS, at least in the subgroup of patients with complement-fixing antiganglioside antibodies.58 Eculizumab, a humanised monoclonal recombinant antibody to complement factor 5, prevents the formation of membrane attack complex and nerve injury in an animal model for GBS.59 This complement inhibitor is therefore a promising new treatment for GBS that is currently being investigated in two RCTs (Inhibition of Complement Activation (Eculizumab) in GBS study (ICA-GBS) in UK and Japanese Eculizumab Trial for GBS (JET-GBS) in Japan).60 ,61
Current personal view: Corticosteroids as single-treatment strategy should be avoided (level of evidence: 1). Methylprednisolone when added to IVIg does not improve long-term outcome but may have a limited effect on short-term outcome (level of evidence: 2).
Treatments other than PE and IVIg
Two small placebo randomised controlled safety studies have reported a non-significant effect of brain-derived neurotrophic factor (BDNF) or interferon β-1a (IFNβ-1a) on disability grade or rate of improvement, respectively.62 ,63 A third small parallel randomised controlled study found a significant effect on improvement of disability grade 8 weeks after the onset of symptoms when patients were treated with a Chinese herbal medicine tripterygium polyglycoside compared with high-dose corticosteroids.64 Another small, open parallel-group study found a similar effect when comparing PE to filtration of cerebrospinal fluid.65 According to the Cochrane review, the numbers in the IFNβ-1a and BDNF studies were too small to exclude clinical relevance and larger sequential RCTs might be more promising.11
Current personal view: At present there is no evidence for the effect of alternative treatments.
Treatment-related fluctuation
Patients with GBS who have received treatment may show a secondary deterioration after initial clinical stabilisation or improvement. This TRF is generally defined as a worsening of at least one grade on the GBS disability scale, or a decrease in Medical Research Council (MRC) sum score after initial stabilisation or improvement within the first 8 weeks after treatment.66 TRFs have been reported in 8–16% of patients with GBS treated with either IVIg or PE.66 ,67 At present it is not possible to predict who may develop a TRF or how long and severe a TRF will be. In a study in children, more TRFs were observed in the 2-day IVIg treatment group (1 g/kg for 2 days) than in the 5-day treatment group (0.4 g/kg for 5 days).26 This may suggest that a shorter treatment regimen is associated with an increased chance to develop a TRF. Clinical deteriorations occurring eight or more weeks after the onset of weakness or for a third time should lead to considering the diagnosis of A-CIDP.67
The mechanism of a TRF has not been elucidated but it has been hypothesised that the effect of treatment is transient while disease activity continues.66 TRFs therefore provide evidence that a treatment in a specific patient is effective, although not lasting long enough, and that the patient will probably respond again after repeating the same treatment. Therefore, it is rational to treat a patient with a TRF with a second course of either IVIg or PE but no RCTs have been conducted to demonstrate the effect.5 The Dutch GBS trial48 showed that the clinical course of patients with a TRF who did not receive a second course, was comparable to those who did, indicating a relatively benign course of a TRF, but the numbers were very small (n=14).66 The current treatment policy often is to re-treat these patients.
Current personal view: We recommend repeating the treatment with IVIg or PE after a TRF, although the effect has not been determined in controlled studies (level of evidence: 4).
Conclusions
Treatment of GBS is complicated by the limited amount of evidence for the treatment effect in various clinical conditions that may frequently occur in GBS. Probably for some of these conditions it will not be possible to determine the effect of treatment in RCTs. Based on the existing evidence from therapeutic studies and our personal experience we have made recommendations for clinical practice (table 5). Future evidence should come from RCTs and from carefully conducted prospective cohort studies in considerable numbers of patients and comparing the outcome after various treatment regimens.
Summary of treatment dilemmas in GBS and recommendations
References
Footnotes
Contributors CV was responsible for the collection of all articles, interpretation and summarising of literature, and writing of the manuscript. PAvD was involved in the interpretation of literature, critical revision of the manuscript and study supervision. BCJ contributed to the manuscript concept and design, interpretation of literature, critical revision of the manuscript and study supervision.
Competing interests PAvD conducted the IVIg/Methylprednisolone RCT and the GRAPH study in patients with GBS and MFS. He is the principal investigator (PI) of the ongoing Second IVIg Dose trial in patients with GBS with a poor prognosis (SID-GBS trial). He is the coauthor of several Cochrane studies in GBS. BCJ received research support from the Netherlands Organization for Health Research and Development, Erasmus MC, Prinses Beatrix Spierfonds, GBS-CIDP Foundation International, CSL-Behring, and Grifols and is the PI of the International GBS Outcome Study (IGOS).
Provenance and peer review Not commissioned; externally peer reviewed.