Abstract
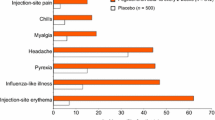
This paper summarizes the worldwide cumulative experience with copolymer 1 (Copaxone) in 857 patients who were enrolled in open-label (n = 586), double-blind (n = 201), and compassionate-use studies (n = 70). The results of a phase III study, including previously unpublished information, are employed to delineate adverse events that occur more frequently among patients treated with copolymer 1 than in placebo-treated controls, and to provide qualitative information. In the cumulative database, patients usually had relapsing-remitting multiple sclerosis and typically received a dose of 20 mg by daily subcutaneous injection for at least 1 year, and occasionally for more than 10 years. Withdrawal rates were 8% for copolymer 1 and 2% for placebo. The most common adverse event was mild injection-site reaction, manifested by erythema, inflammation, and induration. The most remarkable adverse event was a systemic post-injection reaction that occurred in 10% of patients. It was manifested by flushing, chest tightness, palpitations, dyspnea, and anxiety, and was acute and transient. The incidence of adverse events associated with interferon beta, such as flu-like syndrome, depression, hematologic abnormalities, cardiotoxicity, and elevated hepatic enzymes, was not increased among patients treated with copolymer 1. Evaluation of the extensive experience with copolymer 1 confirms that it is well tolerated and suitable for self-administration by patients with multiple sclerosis.
Similar content being viewed by others
References
Abramsky O, Teitelbaum D, Arnon R (1977) Effect of a synthetic polypeptide (Cop-1) on patients with multiple sclerosis and acute disseminated encephalomyelitis: preliminary reports. J Neurol Sci 31:433–438
Bornstein MB, Miller A, Slagle S, et al (1987) A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med 317:408–414
Bornstein MB, Miller A, Slagle S, et al (1991) A placebo-controlled, doubleblind, randomized, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 41:533–539
Cotton P (1994) Options for multiple sclerosis. JAMA 272:1393
Johnson KP, Brooks BR, Cohen JA, et al (1995) Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, doubleblind, placebo-controlled trial. Neurology 45:1268–1276
Teitelbaum D, Meiner Z, Brenner T, et al (1994) Immunological parameters in a multicenter clinical trial of Cop-1 in multiple sclerosis (MS): a 2-year follow-up. Neurology 44 [Suppl 2]:A358-A359
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43:655–661
The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group (1995) Interferon beta-1b in the treat ment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 45:1277–1285
Weiner HL, Hohol MJ, Khoury SJ, et al (1995) Therapy for multiple sclerosis. Neurol Clin 13:173–196
Weinstock-Guttman B, Ransohoff RM, Kinkel RP, et al (1995) The interferons: biological effects, mechanisms of action, and use in multiple sclerosis. Ann Neurol 37:7–15
Wolinsky JS (1995) Copolymer 1: a most reasonable alternative therapy for early relapsing-remitting multiple sclerosis with mild disability. Neurology 45:1245–1247
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korczyn, A.D., Nisipeanu, P. Safety profile of copolymer 1: Analysis of cumulative experience in the United States and Israel. J Neurol 243 (Suppl 1), S23–S26 (1996). https://doi.org/10.1007/BF00873698
Issue Date:
DOI: https://doi.org/10.1007/BF00873698