Abstract
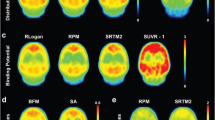
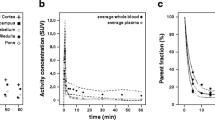
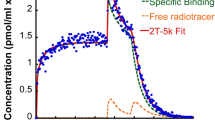
“Delayed” single-photon emission tomograpic (SPET) images after an intravenous bolus injection of iodine-123 iomazenil have been used as a relative map of benzodiazepine receptor binding. We determined the optimal scan time for obtaining such a map and assessed the errors of the map. SPET and blood data from six healthy volunteers and five patients were used. A three-compartment kinetic model was employed in simulation studies and analyses of actual data. The simulation studies suggested that, in the normal brain, the scan time at which a single SPET image best represented the relative receptor binding was 3.0–3.5 h post-injection. This finding was supported by actual data from the volunteers. The simulation studies also suggested that the optimal scan time was not greatly changed by the variability of the input functions, and that the error in the SPET image contrast in the vicinity of the optimal scan time was not increased by changes in the tracer kinetics in the entire brain. The SPET image contrast in the patients at 3.0 h post-injection agreed well with the reference receptor binding estimated by kinetic analysis, with a mean error of 3.6%. These findings support the use of a single SPET image after bolus injection of [123I]iomazenil as a relative map of benzodiazepine receptor binding. For this purpose, a SPET scan time of 3.0-3.5 h post-injection is recommended.
Similar content being viewed by others
References
Hö11 K, Deisenhammer E, Dauth J, Schubiger PA. Imaging benzodiazepine receptors in the human brain by single photon emission computed tomography (SPECT).Nucl Med Biol 1989; 16: 759–763.
Beer HF, Blauenstein PA, Hasler PH, et al. In vitro and in vivo evaluation of iodine- 123-Ro 16-0154: a new imaging agent for SPECT investigations of benzodiazepine receptors.J Nucl Med 1990; 31: 1007–1014.
van Huffelen AC, van Isselt JW van Veelen CWM, et al. Identification of the side of the epileptic focus with123I-iomazenil SPECT. A comparison with18FDG-PET and ictal EEG findings in patients with medically intractable complex partial seizures.Acta Neurochir (Suppl) 1990; 50: 95–99.
Ferstl FJ, Cordes M, Cordes I, et al. 123-I-Iomazenil-SPECT in patients with focal epilepsies - a comparative study with99mTc-HMPAO-SPECT, CT and MR.Adv Exp Med Biol 1991; 287: 405–412.
Schubiger PA, Hasler PH, Beer-Wohlfahrt H, et al. Evaluation of a multicentre study with iomazenil — a benzodiazepine receptor ligand.Nucl Med Commun 1991; 12: 569–582.
Cordes M, Henkes H, Ferstl F, et al. Evaluation of focal epilepsy: a SPECT scanning of 123-iomazenil versus HM-PAO.Am J Neuroradiol 1992; 13: 249–253.
Haldemann RC, Bicik I, Pfeiffer A, et al.123I-iomazenil: a quantitative study of the central benzodiazepine receptor distribution.Nucl Med 1992; 31: 91–97.
Bartenstein P, Ludolph A, Schober O, et al. Benzodiazepine receptors and cerebral blood flow in partial epilepsy.Eur J Nucl Med 1991; 18: 111–118.
Norikiyo T. Evaluation of cortical benzodiazepine receptor imaging SPECT in temporal lobe epilepsy - a comparative study with123I-IMP SPECT.J Jpn Epil Soc 1995; 13: 184–194.
Doi T, Matuda K, Yagi K, Seino S. Clinical usability of123I-iomazenil SPECT for intractable localization-related epilepsies.J Jpn Epil Soc 1995; 13: 184–194.
Tanaka F, Yonekura Y, Ikeda A, et al. Decreased binding potential of benzodiazepine receptors in partial epilepsy measured by I-123 iomazenil SPECT: comparison of late scan and kinetic analysis.J Cereb Blood Flow Metab 1995; 15 Suppl 1: S780.
Torizuka K, Uemura K, Toru M, et al. A phase 3 clinical trial of123I-iomazenil, a new central-type benzodiazepine receptor imaging agent (part 3) — report on clinical usefulness in epilepsy.Jpn J Nucl Med 1996; 33: 319–328.
Hatazawa J, Satoh T, Shimosegawa E, et al. Evaluation of cerebral infarction with iodine-123-iomazenil SPECT.J Nucl Med 1995; 36: 2154–2161.
Hatazawa J, Shimosegawa E, Satoh T, Kanno I, Uemura K. Central benzodiazepine receptor distribution after subcortical hemorrhage evaluated by means of [123I]iomazenil and SPECT.Stroke 1995; 26: 2267–2271.
Hatazawa J, Satoh T, Shimosegawa E, et al. Ischemic damage of central benzodiazepine receptor binding in the cerebral infarction and peri-infarct area studied by means of I-123 ionmazenil.J Cereb Blood Flow Metab 1995; 15 Suppl 1: S127.
Nakagawara J, Sperling B, Takeda R, Suematsu K, Nakamura J, Lassen NA. Incomplete brain infarction of early reperfused, CT/MRI intact cortex in embolic stroke: in vivo evidence by123I-iomazenil.J Cereb Blood Flow Metab 1995; 15 Suppl 1: S131.
Itch Y, Fukuuchi Y, Amano T, Sasaki T, Kubo A, Hashimoto J. Usefulness of SPECT image using ligand to benzodiazepine receptor in patients with cerebral infarction.J Cereb Blood Flow Metab 1995; 15 Suppl 1: S675.
Torizuka K, Uemura K, Toru M, et al. A phase 3 clinical trial of123I-iomazenil, a new central-type benzodiazepine receptor imaging agent (part 4) - report on clinical usefulness in diagnosis of cerebrovascular diseases.Jpn J Nucl Med 1996; 33: 329–344.
Kelly C, Wyper D, Patterson J, et al. GABA activity in Alzheimer's disease: a study using123I-iomazenil and SPECT.J Cereb Blood Flow Metab 1995; 15 Suppl 1: S100.
Kitamura S, Koshi Y, Komiyama T, et al. Benzodiazepine receptor and cerebral blood flow in early Alzheimer's disease — SPECT study uusing123I-iomazenil and123I-IMP.Jpn J Nucl Med 1996; 33: 49–56.
Kawabata K, Tachibana H, Sugita M, et al. Impairment of benzodiazepine receptor in Parkinson's disease evaluated by123I-iomazenil SPECT.Jpn J Nucl Med 1996; 33: 391–397.
Torizuka K, Uemura K, Toru M, et al. A phase 3 clinical trial of123I-iomazenil, a new central-type benzodiazepine receptor imaging agent (part 2) — report on clinical usefulness in diagnosis of degenerative neurological diseases and mental disorders.Jpn J Nucl Med 1996; 33: 303–318.
Schlegel S, Steinert H, Bockisch A, Hahn K, Schlosser R, Benkert O. Decreased benzodiazepine receptor binging in panic disorder measured by iomazenil SPECT.Eur Arch Psychiatry Clin Neurosci 1994; 244: 49–51.
Yonekura Y, Nishizawa S, Tanaka F, et al. Phase I clinical study of123I-iomazenil: a new probe to evaluate central-type benzodiazepine receptor with SPECT.Jpn J Nucl Med 1995; 32: 87–97.
Laruelle M, Baldwin RM, Rattner Z, et al. SPECT quantification of [1231]iomazenil binding to benzodiazepine receptors in nonhuman primates. I. Kinetic modeling of single bolus experiments.J Cereb Blood Flow Metab 1994; 14: 439–452.
Abi-Dargham A, Laruelle M, Seibyl J, et al. SPECT measurement of benzodiazepine receptors in human brain with [123I]iomazenil: kinetic and equilibrium paradigms.J Nucl Med 1994: 35: 228–238.
Abi-Dargham A, Gandelman M, Zoghbi SS, et al. Reproducibility of SPECT measurement of benzodiazepine receptors in human brain with [123I]iomazenil.J Nucl Med 1995; 36: 167–175.
Westera G, Buck A, Burger C, et al. Carbon-11 and iodine-123 labelled iomazenil: a direct PET-SPET comparison.Eur J Nucl Med 1996; 23: 5–12.
Laruelle M, Abi-Dargham A, Rattner Z, et al. SPECT measurement of benzodiazepine receptor number and affinity in primate brain: a constant infusion paradigm with [123I]iomaze-nil.Eur J Phamacol 1993; 230: 119–123.
Laruelle M, Abi-Dargham A, Al-Tikriti MS, et al. SPECT quantification of [1231]iomazenil binding to benzodiazepine receptors in nonhuman primates. 11. Equilibrium analysis of constant infusion experiments and correlation with in vitro parameters.J Cereb Blood Flow Metab 1994; 14: 453–465.
Onishi Y, Yonekura Y, Mukai T et al. Simple quantification of benzodiazepine receptor binding and ligand transport using iodine-123-iomazenil and two SPECT scans.J Nucl Med 1995; 36: 1201–1210.
Onishi Y, Yonekura Y, Nishizawa S, et al: Noninvasive quantification of iodine-123-iomazenil SPECT.J Nucl Med 1996; 37: 374–378.
Carson RE, Channing RG, Blasberg RG, et al. Comparison of bolus and infusion methods for receptor quantification: application to [18F]cyclofoxy and positron emission tomography.J Cereb Blood Flow Metab 1993; 13: 24–42.
Monson EW, Woods SW, Zoghbi S, et al. Receptor binding characterization of the benzodiazepine radioligand125I-Ro 16-0154: potential probe for SPECT brain imaging.Life Sci 1990; 47: 1535–1546.
Koeppe RA, Holthoff VA, Frey KA, et al. Compartmental analysis of [11C]flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography.J Cereb Blood Flow Metab 1991; 11: 735–744.
Mintun MA, Raichle ME, Kilbourn MR, et al. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography.Ann Neurol 1984; 15: 217–227.
Kapouleas I, Alavi A, Alves WM, Gur RE, Weiss DW. Registration of three-dimensional MR and PET images of the human brain without markers.Radiology 1991; 181: 731–739.
Nelder JA, Mead R. A simplex method for function minimization.Computer J 1965; 7: 308–312.
Savic I, Persson A, Roland P, et al. In vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci.Lancet 1988; 30: 863–866.
Rupniak NMJ, Prestwich SA, Horton RW, Jenner P, Marsden CD. Alterations in cerebral glutamic acid decarboxylase and3H-flunitrazepam binding during continuous treatment of rats up to 1 yr with haloperidol, sulpiride or clozapine.J Neural Transm 1978; 68: 113–125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Onishi, Y., Yonekura, Y., Tanaka, F. et al. Delayed image of iodine-123 iomazenil as a relative map of benzodiazepine receptor binding: The optimal scan time. Eur J Nucl Med 23, 1491–1497 (1996). https://doi.org/10.1007/BF01254474
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01254474