Abstract.
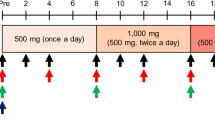
We assessed safety and efficacy of creatine monohydrate (Cr) in myotonic dystrophy (DM1) in a double-blind, cross-over trial. Thirty-four patients with defined DM1 were randomized to receive Cr and placebo for eight weeks (10.6 g day 1–10, 5.3 g day 11–56) in one of 2 treatment sequences. There was no significant improvement using manual and quantitative muscle strength, daily-life activities, and patients' own global assessment comparing verum with placebo administration. Cr supplementation was well tolerated without clinically relevant side effects, but did not result in significant improvement of muscle strength or daily-life activities.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 1 May 2002, Received in revised form: 5 July 2002, Accepted: 12 July 2002
Correspondence to Dr. M. C. Walter
Rights and permissions
About this article
Cite this article
Walter, M., Reilich, P., Lochmüller, H. et al. Creatine monohydrate in myotonic dystrophy . J Neurol 249, 1717–1722 (2002). https://doi.org/10.1007/s00415-002-0923-x
Issue Date:
DOI: https://doi.org/10.1007/s00415-002-0923-x