Abstract
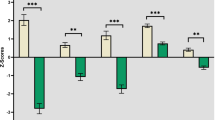
In order to determine the relationship between regional neuropathology and severity of clinical features in Richardson’s syndrome (PSP-RS), the following hypotheses were tested: (1) executive dysfunction relates to prefrontal pathology; (2) language difficulties to pathology in Broca’s area and/or the perirhinal cortex; and (3) visuospatial impairment to pathology in the supramarginal region. A prospectively studied case series of brain donors at a specialist clinic in Addenbrooke’s Hospital Cambridge, UK, were examined. All those fulfilling postmortem criteria for PSP-RS and their last cognitive assessment within 24 months of death (N = 11/25) were included. The degree of regional neuronal loss and neuronal tau deposition across a number of cortical brain regions was performed and compared to 10 age- and sex-matched controls from the Sydney Brain Bank. Stepwise multiple linear regressions were used to determine the neuropathological correlates to cognitive scores and revealed the following. Executive dysfunction, as indexed by letter fluency, related to the degree of tau deposition in the superior frontal gyrus and supramarginal cortices (p < 0.020), language deficits related to neuron loss in the perirhinal gyrus (p < 0.001) and tau deposition in Broca’s area (p = 0.020), while visuospatial dysfunction and global cognitive impairment related to tau deposition in the supramarginal gyrus (p < 0.007). The severity of cognitive deficits relate to regional cortical tau deposition in PSP-RS, although language impairment related to neuronal loss in the perirhinal region. Global cognitive dysfunction related most to the severity of tau deposition in the supramarginal gyrus warranting further research on the role of this brain region in PSP-RS.


Similar content being viewed by others
References
Agosta F, Kostic VS, Galantucci S, Mesaros S, Svetel M, Pagani E, Stefanova E, Filippi M (2010) The in vivo distribution of brain tissue loss in Richardson’s syndrome and PSP-parkinsonism: a VBM-DARTEL study. Eur J Neurosci 32:640–647
Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S (2007) Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68:1515–1523
Bak TH, Caine D, Hearn VC, Hodges JR (2006) Visuospatial functions in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 77:454–456
Bak TH, Crawford LM, Hearn VC, Mathuranath PS, Hodges JR (2005) Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA). Neurocase 11:268–273
Bak TH, Hodges JR (2003) Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. J Neurolinguist 16:169–181
Bak TH, O’Donovan DG, Xuereb JH, Boniface S, Hodges JR (2001) Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain 124:103–120
Bergeron C, Pollanen MS, Weyer L, Lang AE (1997) Cortical degeneration in progressive supranuclear palsy. A comparison with cortical-basal ganglionic degeneration. J Neuropathol Exp Neurol 56:726–734
Bigio EH, Brown DF, CLr White (1999) Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol 58:359–364
Bird CM, Papadopoulou K, Ricciardelli P, Rossor MN, Cipolotti L (2004) Monitoring cognitive changes: psychometric properties of six cognitive tests. Br J Clin Psychol 43:197–210
Bishop DVM (1983) The Test for Reception of Grammar. In: The Author. Age and Cognitive Performance Research Centre, University of Manchester, UK
Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, Agid Y, Ludolph A, Bensimon G, Payan C, Leigh NP (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133:2382–2393
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C (2005) Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 128:1259–1266
Cordato NJ, Pantelis C, Halliday GM, Velakoulis D, Wood SJ, Stuart GW, Currie J, Soo M, Olivieri G, Broe GA, Morris JG (2002) Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain 125:789–800
Croot K, Hodges JR, Patterson K (1999) Evidence for impaired sentence comprehension in early Alzheimer’s disease. J Int Neuropsychol Soc 5:393–404
Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR (2009) The neural basis of semantic memory: evidence from semantic dementia. Neurobiol Aging 30:2043–2052
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dudas RB, Berrios GE, Hodges JR (2005) The Addenbrooke’s cognitive examination (ACE) in the differential diagnosis of early dementias versus affective disorder. Am J Geriatr Psychiatry 13:218–226
Esmonde T, Giles E, Gibson M, Hodges JR (1996) Neuropsychological performance, disease severity, and depression in progressive supranuclear palsy. J Neurol 243:638–643
Ghosh BC, Rowe JB, Calder AJ, Hodges JR, Bak TH (2009) Emotion recognition in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 80:1143–1145
Golbe LI (1997) Medical Advisory Board of the Society for Progressive Supranuclear Palsy. A clinical rating scale and staging system for progressive supranuclear palsy. Neurology 48:A326
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain 130:1552–1565
Halliday GM, Hardman CD, Cordato NJ, Hely MA, Morris JG (2000) A role for the substantia nigra pars reticulata in the gaze palsy of progressive supranuclear palsy. Brain 123(Pt 4):724–732
Hanihara T, Amano N, Takahashi T, Nagatomo H, Yagashita S (1995) Distribution of tangles and threads in the cerebral cortex in progressive supranuclear palsy. Neuropathol Appl Neurobiol 21:319–326
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hayden BY, Platt ML (2010) Neurons in anterior cingulate cortex multiplex information about reward and action. J Neurosci 30:3339–3346
Hodges JR (2007) Cognitive assessment for clinicians. Oxford University Press, Oxford
Hof PR, Delacourte A, Bouras C (1992) Distribution of cortical neurofibrillary tangles in progressive supranuclear palsy: a quantitative analysis of six cases. Acta Neuropathol (Berl) 84:45–51
Howard D, Patterson K (1992) Pyramids and palm trees: a test of semantic access from pictures and words. Thames Valley Test Company, Bury St Edmunds
Jellinger KA (2008) Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis 5:339–346
Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, Petersen RC, Dickson DW (2005) Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 11:283–296
Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR Jr (2008) Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 29:280–289
Kersaitis C, Halliday GM, Kril JJ (2004) Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol 108:515–523
Kril JJ, Halliday GM, Svoboda MD, Cartwright H (1997) The cerebral cortex is damaged in chronic alcoholics. Neuroscience 79:983–998
Litvan I (1994) Cognitive disturbances in progressive supranuclear palsy. J Neural Transm Suppl 42:69–78
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK (2003) Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18:467–486
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR (2000) A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 55:1613–1620
Millar D, Griffiths P, Zermansky AJ, Burn DJ (2006) Characterizing behavioral and cognitive dysexecutive changes in progressive supranuclear palsy. Mov Disord 21:199–207
Rushworth MF, Paus T, Sipila PK (2001) Attention systems and the organization of the human parietal cortex. J Neurosci 21:5262–5271
Schofield EC, Hodges JR, Macdonald V, Cordato NJ, Kril JJ, Halliday GM (2010) Cortical atrophy differentiates Richardson’s syndrome from the parkinsonian form of progressive supranuclear palsy. Mov Disord. doi:10.1002/mds.23295
Silk TJ, Bellgrove MA, Wrafter P, Mattingley JB, Cunnington R (2010) Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. Neuroimage 53:718–724
Verny M, Duyckaerts C, Agid Y, Hauw J (1996) The significance of cortical pathology in progressive supranuclear palsy. Clinico-pathological data in 10 cases. Brain 119:1123–1136
Warrington EK, James M (1991) The visual object and space perception battery. Thames Valley Test Company, Bury St. Edmunds
Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ (2005) Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128:1247–1258
Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T (2007) Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130:1566–1576
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Acknowledgments
Tissues were received from the Cambridge Brain Bank, which is supported by the Medical Research Council, UK and the Sydney Brain Bank, which is supported by the National Health and Medical Research Council (NHMRC) of Australia (Enabling Grant 282933 & 052009), Neuroscience Research Australia and the University of New South Wales. The study was supported through research grants from the PSP Association. ES was supported by a NHMRC biomedical postgraduate research scholarship 300672. JH was supported by an ARC Federation Fellowship. GH was supported by a NHMRC Senior Principal Research Fellowships 350827 & 630434.
Conflict of interest
The authors declare that they have no conflict of interest and have no further financial disclosures to make.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schofield, E.C., Hodges, J.R., Bak, T.H. et al. The relationship between clinical and pathological variables in Richardson’s syndrome. J Neurol 259, 482–490 (2012). https://doi.org/10.1007/s00415-011-6205-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6205-8