Abstract
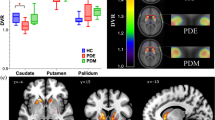
Tremor dominant (TD) and akinetic-rigid type (ART) are two motor subtypes of Parkinson’s disease associated with different disease progression and neurochemical/neuropathological features. The role of presynaptic nigrostriatal dopaminergic damage is still controversial, poorly explored, and only assessed in medicated patients. In this study, we investigated with FP-CIT SPECT the striatal dopamine transporter (DAT) availability in drug-naïve PD patients with ART and TD phenotypes. Fifty-one de novo, drug-naïve patients with PD underwent FP-CIT SPECT studies. Patients were evaluated with Unified Parkinson’s Disease Rating Scale (UPDRS) part III and Hoehn and Yahr scale (H&Y) and divided into ART (24/51) and TD (27/51) according to UPDRS part III. ART and TD patients were not different with regard to age, gender, and disease duration. However, compared to TD, ART patients presented higher UPDRS part III (p = 0.01) and H&Y (p = 0.02) and lower DAT availability in affected and unaffected putamen (p = 0.008 and p = 0.007, respectively), whereas no differences were found in caudate. Moreover, in the whole group of patients, rigidity and bradykinesia, but not tremor scores of UPDRS part III were significantly related to FP-CIT binding in the putamen. These results suggest that in newly diagnosed drug-naïve PD patients DAT availability might be different between ART and TD in relation to different disease severity.


Similar content being viewed by others
References
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39. doi:10.1001/archneur.56.1.33
Lewis SJG, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA (2005) Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 76(3):343–348. doi:10.1136/jnnp.2003.033530
Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D (2006) Changes in motor subtype and risk for incident dementia in parkinson’s disease. Mov Disord 21(8):1123–1130. doi:10.1002/mds.20897
Burn DJ, Rowan EN, Allan LM, Molloy S, O’Brien JT, McKeith IG (2006) Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 77(5):585–589. doi:10.1136/jnnp.2005.081711
Oh JY, Kim YS, Choi BH, Sohn EH, Lee AY (2009) Relationship between clinical phenotypes and cognitive impairment in Parkinson’s disease. Arch Gerontol Geriatr 49(3):351–354. doi:10.1016/j.archger.2008
Reijnders JS, Ehrt U, Lousberg R, Aarsland D, Leentjens AF (2009) The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat Disord 15(5):379–382. doi:10.1016/j.parkreldis.2008.09.003
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 72(4):499–504. doi:10.1001/jamaneurol.2013.6233
Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O (2008) Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology 70(16 Pt 2):1403–1410. doi:10.1212/01.wnl.0000285082.18969.3a
Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ (2009) A clinico-pathological study of subtypes in Parkinson’s disease. Brain 132(Pt 11):2947–2957. doi:10.1093/brain/awp234
Spiegel J, Hellwig D, Samnick S, Jost W, Möllers MO, Fassbeneder K, Kirsch CM, Dillmann U (2007) Striatal FP-CIT uptake differs in the subtypes of early Parkinson’s disease. J Neural Transm 114(3):331–335. doi:10.1007/s00702-006-0518-2
Rossi C, Frosini D, Volterrani D, De Feo P, Unti E, Nicoletti V, Kiferle L, Bonuccelli U, Ceravolo R (2010) Differences in nigro-striatal impairment in clinical variants of early Parkinson’s disease: evidence from a FP-CIT SPECT study. Eur J Neurol 17(4):626–630. doi:10.1111/j.1468-1331.2009.02898.x
Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L (2011) Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT single photon emission computed tomography. Mov Disord 26(3):416–423. doi:10.1002/mds.23468
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinic- pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80(5):496–503. doi:10.1212/WNL.0b013e31827f0fd1
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676. doi:10.1212/01.wnl.0000324625.00404.15
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, Brooks D, Burn DJ, Colosimo C, Fanciulli A, Ferreira J, Gasser T, Grandas F, Kanovsky P, Kostic V, Kulisevsky J, Oertel W, Poewe W, Reese JP, Relja M, Ruzicka E, Schrag A, Seppi K, Taba P, Vidailhet M (2013) EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 20(1):16–34. doi:10.1111/ene.12022
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimer’s Dis 9(3 Suppl):417–423
Fahn S, Elton RL, UPDRS program members (1987) Unified Parkinson’s disease rating scale. In: Recent developments in Parkinson’s disease, vol. 2. Florham Park. NJ: Macmillan Healthcare information. pp 153–163
Varrone A, Pellecchia MT, Amboni M, Sansone V, Salvatore E, Ghezzi D, Garavaglia B, Brice A, Brunetti A, Bonavita V, De Michele G, Salvatore M, Pappatà S, Barone P (2004) Imaging of dopaminergic dysfunction with 123I-FP-CIT SPECT in early-onset parkin disease. Neurology 63(11):2097–2103. doi:10.1212/01.WNL.0000145765.19094.94
Song IU, Chung YA, Oh JK, Chung SW (2014) An FP-CIT PET comparison of the difference in dopaminergic neuronal loss in subtypes of early Parkinson’s disease. Acta Radiol 55(3):366–371. doi:10.1177/0284185113498075
Tissingh G, Bergmans P, Booij J, Winogrodzka A, van Royen EA, Stoof JC, Wolters EC (1998) Drug-naïve patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]-b-CIT-SPECT. J Neurol 245:14–20
Brooks DJ, Playford ED, Ibanez V, Sawle GV, Thompson PD, Findley LJ, Marsden CD (1992) Isolated tremor and disruption of the nigrostriatal dopaminergic system: an 18F-dopa PET study. Neurology 42(8):1554–1560
Isaias IU, Benti R, Cilia R, Canesi M, Marotta G, Gerundini P, Pezzoli G, Antonini A (2007) [123I]FP-CIT striatal binding in early Parkinson’s disease patients with tremor vs. akinetic-rigid onset. NeuroReport 18(14):1499–1502
Pirker W (2003) Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord 18(Suppl 7):S43–S51. doi:10.1002/mds.10579
Jellinger KA (1999) Post mortem studies in Parkinson’s disease—is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 56:1–29
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50(6):743–755
Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69(2):269–281. doi:10.1002/ana.22361
Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, Mailman RB, Huang X (2011) Differential involvement of striato- and cerebello-thalamo- cortical pathways in tremor-and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177:230–239. doi:10.1016/j.neuroscience.2010.12.060
Mure H, Hirano S, Tang CC, Isaias IU, Antonini A, Ma Y, Dhawan V, Eidelberg D (2011) Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54(2):1244–1253. doi:10.1016/j.neuroimage.2010.09.028
Carr J (2002) Tremor in Parkinson’s disease. Parkinsonism Relat Disord 8(4):223–234. doi:10.1016/S1353-8020(01)00037-2
Caretti V, Stoffers D, Winogrodzka A, Isaias IU, Costantino G, Pezzoli G, Ferrarese C, Antonini A, Wolters EC, Booij J (2008) Loss of thalamic serotonin transporters in early drug-naïve Parkinson’s disease patients is associated with tremor: an [(123)I]beta-CIT SPECT study. J Neural Transm 115(5):721–729. doi:10.1007/s00702-007-0015-2
Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ (2003) Tremor in Parkinson’s disease and serotoninergic dysfunction. Neurology 60:601–605. doi:10.1212/01.WNL.0000031424.51127.2B
Halliday GM, Holton JL, Revesz T, Dickson DW (2011) Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122(2):187–204. doi:10.1007/s00401-011-0852-9
Kaasinen V, Kinos M, Joutsa J, Seppanen M, Noponen T (2014) Differences in striatal dopamine transporter density between tremor dominant and non-tremor Parkinson’s disease. Eur J Nucl Med Mol Imaging. doi:10.1007/s00259-014-2796-5
Acknowledgments
The present work was partly supported by MEdical Research in ITaly (MERIT) Project No_ RBNE08LN4P. The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no HEALTH-F2- 2011-278850 (INMiND).
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
The present study recevied approval from the ethics committee of “Federico II” University Hospital and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All patients gave appropriate informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moccia, M., Pappatà, S., Picillo, M. et al. Dopamine transporter availability in motor subtypes of de novo drug-naïve Parkinson’s disease. J Neurol 261, 2112–2118 (2014). https://doi.org/10.1007/s00415-014-7459-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7459-8