Abstract
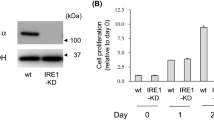
Transmembrane protein 132A (TMEM132A) is a novel GRP78 binding protein that we recently discovered. However, the biological functions of TMEM132A are merely characterized because it does not encode any known structural domains. In this study, we down regulated intrinsic TMEM132A by RNA interference and identified a variety of genes that fluctuated during TMEM132A gene silencing using microarray analysis. TMEM132A-knockdown in Neuro2a cells caused neurite-like projection without any stimuli and enhanced the expression of ATF6 mRNA, an ER stress transducer, and GADD153 mRNA, a stress inducible gene. Under serum-deprived condition, TMEM132A-knockdown cells gradually retarded neurite-like projection and decreased cell viability. Moreover, TMEM132A knockdown markedly induced GADD153 expression due to serum starvation without affecting the level of cleaved caspase-3. Our data suggest that TMEM132A is an important factor of cell survival in regulating certain ER stress-related gene expression in neuronal cells.




Similar content being viewed by others
Abbreviations
- ATF6:
-
Activating transcription factor 6
- GADD153:
-
Growth arrest and DNA damage-inducible protein 153
- GRP78:
-
Glucose-regulated protein, 78 kDa
- siRNA:
-
Small interference RNA
- TMEM132A:
-
Transmembrane protein 132A
References
Oh-hashi K, Naruse Y, Amaya F, Shimosato G, Tanaka M (2003) Cloning and characterization of a novel GRP78-binding protein in the rat brain. J Biol Chem 278:10531–10537
Wright KM, Vaughn AE, Deshmukh M (2007) Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell Death Differ 14:625–633
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441:513–517
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403:98–103
Oh-hashi K, Hirata Y, Koga H, Kiuchi K (2006) GRP78-binding protein regulates cAMP-induced glial fibrillary acidic protein expression in rat C6 glioblastoma cells. FEBS Lett 580:3943–3947
Zhu C, Johansen FE, Prywes R (1997) Interaction of ATF6 and serum response factor. Mol Cell Biol 17:4957–4966
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594
Ron D, Habener JF (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6:439–453
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Gustincich S, Vatta P, Goruppi S, Wolf M, Saccone S, Della Valle G, Baggiolini M, Schneider C (1999) The human serum deprivation response gene (SDPR) maps to 2q32-q33 and codes for a phosphatidylserine-binding protein. Genomics 57:120–129
Yun JS, Rust JM, Ishimaru T, Díaz E (2007) A novel role of the Mad family member Mad3 in cerebellar granule neuron precursor proliferation. Mol Cell Biol 27:8178–8189
Luyten A, Mortier E, Van Campenhout C, Taelman V, Degeest G, Wuytens G, Lambaerts K, David G, Bellefroid EJ, Zimmermann P (2008) The postsynaptic density 95/disc-large/zona occludens protein syntenin directly interacts with frizzled 7 and supports noncanonical Wnt signaling. Mol Biol Cell 19:1594–1604
Walkley SU, Suzuki K (2004) Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta 1685:48–62
Armentano M, Filosa A, Andolfi G, Studer M (2006) COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development 133:4151–4162
Taylor WR, Stark GR (2001) Regulation of the G2/M transition by p53. Oncogene 20:1803–1815
Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG (2002) Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA 99:15920–15925
Rutkowski DT, Kaufman RJ (2003) All road to ATF4. Dev Cell 4:442–444
Namba T, Ishihara T, Tanaka K, Hoshino T, Mizushima T (2007) Transcriptional activation of ATF6 by endoplasmic reticulum stressors. Biochem Biophys Res Commun 355:543–548
Qiao D, Im E, Qi W, Martinez JD (2002) Activator protein-1 and CCAAT/enhancer-binding protein mediated GADD153 expression is involved in deoxycholic acid-induced apoptosis. Biochim Biophys Acta 1583:108–116
Oh-hashi K, Maehara K, Isobe K (2004) Hydrogen peroxide induces GADD153 in Jurkat cells through the protein kinase C-dependent pathway. Redox Rep 9:173–178
van der Sanden MH, Meems H, Houweling M, Helms JB, Vaandrager AB (2004) Induction of CCAAT/enhancer-binding protein (C/EBP)-homologous protein/growth arrest and DNA damage-inducible protein 153 expression during inhibition of phosphatidylcholine synthesis is mediated via activation of a C/EBP-activating transcription factor-responsive element. J Biol Chem 279:52007–52015
Li LY, Luo X, Wang X (2001) Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412:95–99
Torriglia A, Leprêtre C, Padrón-Barthe L, Chahory S, Martin E (2008) Molecular mechanism of L-DNase II activation and function as a molecular switch in apoptosis. Biochem Pharmacol 76:1490–1502
Boland B, Campbell V (2004) Aβ-mediated activation of the apoptotic cascade in cultured cortical neurones: a role for cathepsin-L. Neurobiol Aging 25:83–91
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172:454–469
Steiger-Barraissoul S, Rami A (2009) Serum deprivation induced autophagy and predominantly an AIF-dependent apoptosis in hippocampal HT22 neurons. Apoptosis 14:1274–1288
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, Lu PD, Marciniak SJ, Ron D, Przedborski S, Kholodilov N, Greene LA, Burke RE (2005) CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem 95:974–986
Cortes-Canteli M, Pignatelli M, Santos A, Perez-Castillo A (2002) CCAAT/enhancer-binding protein β plays a regulatory role in differentiation and apoptosis of neuroblastoma cells. J Biol Chem 277:5460–5467
Kögel D, Svensson B, Copanaki E, Anguissola S, Bonner C, Thurow N, Gudorf D, Hetschko H, Müller T, Peters M, König HG, Prehn JH (2006) Induction of transcription factor CEBP homology protein mediates hypoglycaemia-induced necrotic cell death in human neuroblastoma cells. J Neurochem 99:952–964
Acknowledgments
This study was supported in part by grant-in-aid for young scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh-hashi, K., Imai, K., Koga, H. et al. Knockdown of transmembrane protein 132A by RNA interference facilitates serum starvation-induced cell death in Neuro2a cells. Mol Cell Biochem 342, 117–123 (2010). https://doi.org/10.1007/s11010-010-0475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0475-9