Abstract
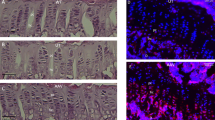
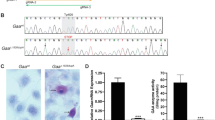
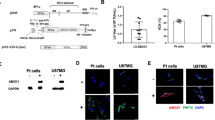
As more functional redundancy in mammalian cells is discovered, enhanced expression of genes involved in alternative pathways may become an effective form of gene therapy. X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal disorder with impaired very-long-chain fatty acid metabolism. The X-ALD gene encodes a peroxisomal membrane protein (ALDP) that is part of a small family of related peroxisomal membrane proteins. We show that 4-phenylbutyrate treatment of cells from both X-ALD patients and X-ALD knockout mice results in decreased levels of and increased β-oxidation of very-long-chain fatty acids; increased expression of the peroxisomal protein ALDRP; and induction of peroxisome proliferation. We also demonstrate that ALDP and ALDRP are functionally related, by ALDRP cDNA complementation of X-ALD fibroblasts. Finally, we demonstrate the in vivo efficacy of dietary 4-phenylbutyrate treatment through its production of a substantial reduction of very-long-chain fatty acid levels in the brain and adrenal glands of X-ALD mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cooke, J., Nowak, M.A., Boerlijst, M. & Maynard-Smith, J. Evolutionary origins and maintenance of redundant gene expression during metazoan development. Trends Genet. 13, 360– 364 (1997).
Thomas, J.H. Thinking about genetic redundancy. Trends Genet. 9, 395–399 (1993).
Charache, S. et al. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc. Natl. Acad. Sci. USA 80, 4842– 4846 (1983).
Dover, G.J. et al. Hydroxyurea induction of hemoglobin F production in sickle cell disease: relationship between cytotoxicity and F cell production. Blood 67, 735–738 ( 1986).
Perrine, S.P. et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders [see comments]. N. Engl. J. Med. 328, 81–86 ( 1993).
Dover, G.J., Brusilow, S. & Charache, S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood 84, 339–343 (1994).
Tinsley, J.M. et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene [see comments]. Nature 384, 349–353 (1996).
Roush, W. Backup gene may help muscles help themselves [news]. Science 276, 35 (1997).
Wanders, R.J. et al. Direct demonstration that the deficient oxidation of very long chain fatty acids in X-linked adrenoleukodystrophy is due to an impaired ability of peroxisomes to activate very long chain fatty acids. Biochem. Biophys. Res. Commun. 153, 618– 624 (1988).
Lazo, O., Contreras, M., Bhushan, A., Stanley, W. & Singh, I. Adrenoleukodystrophy: impaired oxidation of fatty acids due to peroxisomal lignoceroyl-CoA ligase deficiency. Arch. Biochem. Biophys. 270, 722–728 (1989).
Moser, H.W., Smith, K.D. & Moser, A.B. in The Metabolic Basis of Inherited Disease 7th edn. (eds. Scirver, C.R., Baeudet, A.L., Sly, W.S. & Valle, D.) 4256–4300 (McGraw Hill, New York, 1995).
Powers, J.M. Adreno-leukodystrophy (adreno-testiculo-leukomyelo-neuropathic-complex). Clin. Neuropathol. 4, 181–199 (1985).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Kemp, S. et al. Identification of a two base pair deletion in five unrelated families with adrenoleukodystrophy: a possible hot spot for mutations. Biochem. Biophys. Res. Commun. 202, 647– 653 (1994).
Kok, F. et al. Mutational analysis of patients with X-linked adrenoleukodystrophy. Hum. Mutat. 6, 104–115 (1995).
Mosser, J. et al. The gene responsible for adrenoleukodystrophy encodes a peroxisomal membrane protein. Hum. Mol. Genet. 3, 265 –271 (1994).
Ligtenberg, M.J. et al. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am. J. Hum. Genet. 56, 44– 50 (1995).
Feigenbaum, V. et al. Mutational and protein analysis of patients and heterozygous women with X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 58, 1135–1144 (1996).
Krasemann, E.W., Meier, V., Korenke, G.C., Hunneman, D.H. & Hanefeld, F. Identification of mutations in the ALD-gene of 20 families with adrenoleukodystrophy/adrenomyeloneuropathy. Hum. Genet. 97, 194–197 (1996).
Cartier, N. et al. Retroviral-mediated gene transfer corrects very-long-chain fatty acid metabolism in adrenoleukodystrophy fibroblasts. Proc. Natl. Acad. Sci. USA 92, 1674–1678 (1995).
Braiterman, L.T. et al. Suppression of peroxisomal membrane protein defects by peroxisomal ATP binding cassette (ABC) proteins. Hum. Mol. Genet. 7, 239–247 ( 1998).
Lombard-Platet, G., Savary, S., Sarde, C.O., Mandel, J.L. & Chimini, G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. USA 93, 1265– 1269 (1996).
Holzinger, A., Kammerer, S., Berger, J. & Roscher, A.A. cDNA cloning and mRNA expression of the human adrenoleukodystrophy related protein (ALDRP), a peroxisomal ABC transporter. Biochem. Biophys. Res. Commun. 239, 261–264 (1997).
Kamijo, K., Taketani, S., Yokota, S., Osumi, T. & Hashimoto, T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 265, 4534–4540 (1990).
Gartner, J., Moser, H. & Valle, D. Mutations in the 70K peroxisomal membrane protein gene in Zellweger syndrome. Nature Genet. 1, 16–23 (1992).
Shani, N., Jimenez-Sanchez, G., Steel, G., Dean, M. & Valle, D. Identification of a fourth half ABC transporter in the human peroxisomal membrane. Hum. Mol. Genet. 6, 1925–1931 ( 1997).
Holzinger, A., Kammerer, S. & Roscher, A.A. Primary structure of human PMP69, a putative peroxisomal ABC-transporter. Biochem. Biophys. Res. Commun. 237 , 152–157 (1997).
Boles, D.J., Craft, D.A., Padgett, D.A., Loria, R.M. & Rizzo, W.B. Clinical variation in X-linked adrenoleukodystrophy: fatty acid and lipid metabolism in cultured fibroblasts. Biochem. Med. Metab. Biol. 45, 74–91 (1991).
Lu, J.F. et al. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 94, 9366– 9371 (1997).
Loes, D.J. et al. Childhood cerebral form of adrenoleukodystrophy: short-term effect of bone marrow transplantation on brain MR observations. AJNR Am. J. Neuroradiol. 15, 1767– 1771 (1994).
Moser, H.W. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 120, 1485–1508 ( 1997).
Poulos, A., Gibson, R., Sharp, P., Beckman, K. & Grattan-Smith, P. Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo's oil. Ann. Neurol. 36, 741–746 (1994).
Rasmussen, M., Moser, A.B., Borel, J., Khangoora, S. & Moser, H.W. Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's oil). Neurochem. Res. 19, 1073–1082 ( 1994).
Maestri, N.E., Brusilow, S.W., Clissold, D.B. & Bassett, S.S. Long-term treatment of girls with ornithine transcarbamylase deficiency. N. Engl. J. Med. 335, 855–859 (1996).
Carstea, E.D., Murray, G.J. & O'Neill, R.R. Molecular and functional characterization of the murine glucocerebrosidase gene. Biochem. Biophys. Res. Commun. 184, 1477–1483 (1992).
Deng, G., Liu, G., Hu, L., Gum, J.R., Jr. & Kim, Y.S. Transcriptional regulation of the human placental-like alkaline phosphatase gene and mechanisms involved in its induction by sodium butyrate. Cancer Res. 52, 3378–3383 (1992).
Fregeau, C.J., Helgason, C.D. & Bleackley, R.C. Two cytotoxic cell proteinase genes are differentially sensitive to sodium butyrate. Nucleic Acids Res. 20 , 3113–3119 (1992).
Liu, L., Hudgins, W.R., Miller, A.C., Chen, L.C. & Samid, D. Transcriptional upregulation of TGF-alpha by phenylacetate and phenylbutyrate is associated with differentiation of human melanoma cells. Cytokine 7, 449– 456 (1995).
Albet, S. et al. Fenofibrate differently alters expression of genes encoding ATP-binding transporter proteins of the peroxisomal membrane. FEBS Lett. 405, 394–397 (1997).
Rubenstein, R.C., Egan, M.E. & Zeitlin, P.L. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J. Clin. Invest. 100, 2457–2465 (1997).
Rubenstein, R.C. & Zeitlin, P.L. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am. J. Respir. Crit. Care. Med. 157, 484 –490 (1998).
Watkins, P.A. et al. Altered expression of ALDP in X-linked adrenoleukodystrophy. Am. J. Hum. Genet. 57, 292– 301 (1995).
Kemp, S. et al. ALDP expression in fibroblasts of patients with X-linked adrenoleukodystrophy. J. Inherit. Metab. Dis. 19, 667– 674 (1996).
Reddy, J.K., Suga, T., Mannaerts, G.P., Lazarow, P.B. & Subramani, S. in Annals of the New York Academy of Sciences Vol. 804 (eds. Boland, B., Cullinan, J., Cullinan, D.M. & Garry, M.L.) 801 (New York Academy of Sciences, New York, 1996).
Pineau, T. et al. Activation of a human peroxisome proliferator-activated receptor by the antitumor agent phenylacetate and its analogs. Biochem. Pharmacol. 52, 659–667 ( 1996).
Aoyama, T., Souri, M., Kamijo, T., Ushikubo, S. & Hashimoto, T. Peroxisomal acyl-coenzyme A oxidase is a rate-limiting enzyme in a very-long-chain fatty acid beta-oxidation system. Biochem. Biophys. Res. Commun. 201, 1541– 1547 (1994).
Fouquet, F. et al. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 3 , 271–285 (1997).
Gould, S.J., Krisans, S., Keller, G.A. & Subramani, S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J. Cell Biol. 110, 27–34 ( 1990).
Mihalik, S.J. et al. Identification of PAHX, a Refsum disease gene. Nature Genet. 17, 185–189 (1997).
Ohba, T. et al. The structure of the human sterol carrier protein X/sterol carrier protein 2 gene (SCP2). Genomics 24, 370– 374 (1994).
Sakai, Y. et al. The Candida boidinii peroxisomal membrane protein Pmp30 has a role in peroxisomal proliferation and is functionally homologous to Pmp27 from Saccharomyces cerevisiae. J. Bacteriol. 177, 6773–6781 (1995).
Erdmann, R. & Blobel, G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 128, 509– 523 (1995).
Ram, Z. et al. Growth inhibition, tumor maturation, and extended survival in experimental brain tumors in rats treated with phenylacetate. Cancer Res. 54, 2923–2927 (1994).
Moser, H.W. & Moser, A.B. in Techniques in Diagnostic Biochemical Genetics: A Laboratory Manual (ed. A., H.F.) 177– 191 (Wiley-Liss, New York, 1991).
Watkins, P.A., Ferrell, E.V., Jr., Pedersen, J.I. & Hoefler, G. Peroxisomal fatty acid beta-oxidation in HepG2 cells. Arch. Biochem. Biophys. 289, 329–336 ( 1991).
Zenger-Hain, J., Craft, D.A. & Rizzo, W.B. in New Developments in Fatty Acid Oxidation (eds Coates, P.M. & Tanada, K.) 339–407 (Wiley-Liss, New York, 1992).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Acknowledgements
We thank H. Moser for his advice and continuing interest in this study, and S. Brusilow for discussions. We thank A. Liu, S. Wang, S. Bergin, S. Khangoora and D. Gordon for technical assistance. For review of the manuscript, we thank H. Moser, S. Brusilow, S. Gould, D. Valle and P. Zeitlin. This work was supported by grants from the National Institutes of Health, HD10981, HD24061, DK51149, and GM07814, the Myelin Project and the United Leukodystrophy Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kemp, S., Wei, HM., Lu, JF. et al. Gene redundancy and pharmacological gene therapy: Implications for X-linked adrenoleukodystrophy. Nat Med 4, 1261–1268 (1998). https://doi.org/10.1038/3242
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3242
This article is cited by
-
Targeted Brain Delivery of Dendrimer-4-Phenylbutyrate Ameliorates Neurological Deficits in a Long-Term ABCD1-Deficient Mouse Model of X-Linked Adrenoleukodystrophy
Neurotherapeutics (2023)
-
Adrenoleukodystrophy – neuroendocrine pathogenesis and redefinition of natural history
Nature Reviews Endocrinology (2016)
-
Thyroid Hormone Signaling in Oligodendrocytes: from Extracellular Transport to Intracellular Signal
Molecular Neurobiology (2016)
-
Innovative strategies to treat protein misfolding in inborn errors of metabolism: pharmacological chaperones and proteostasis regulators
Journal of Inherited Metabolic Disease (2014)
-
The peroxisome: an update on mysteries
Histochemistry and Cell Biology (2012)