Abstract
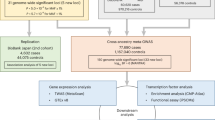
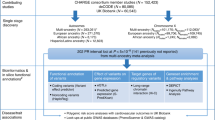
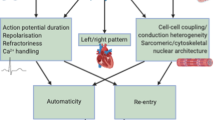
Atrial fibrillation is a highly prevalent arrhythmia and a major risk factor for stroke, heart failure and death1. We conducted a genome-wide association study (GWAS) in individuals of European ancestry, including 6,707 with and 52,426 without atrial fibrillation. Six new atrial fibrillation susceptibility loci were identified and replicated in an additional sample of individuals of European ancestry, including 5,381 subjects with and 10,030 subjects without atrial fibrillation (P < 5 × 10−8). Four of the loci identified in Europeans were further replicated in silico in a GWAS of Japanese individuals, including 843 individuals with and 3,350 individuals without atrial fibrillation. The identified loci implicate candidate genes that encode transcription factors related to cardiopulmonary development, cardiac-expressed ion channels and cell signaling molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fuster, V. et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114, e257–e354 (2006).
Ellinor, P.T. et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat. Genet. 42, 240–244 (2010).
Benjamin, E.J. et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat. Genet. 41, 879–881 (2009).
Gudbjartsson, D.F. et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 448, 353–357 (2007).
Gudbjartsson, D.F. et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat. Genet. 41, 876–878 (2009).
Pfeufer, A. et al. Genome-wide association study of PR interval. Nat. Genet. 42, 153–159 (2010).
Holm, H. et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat. Genet. 42, 117–122 (2010).
Lubitz, S.A. et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. J. Am. Med. Assoc. 304, 2263–2269 (2010).
Bergwerff, M. et al. Loss of function of the Prx1 and Prx2 homeobox genes alters architecture of the great elastic arteries and ductus arteriosus. Virchows Arch. 436, 12–19 (2000).
Ihida-Stansbury, K. et al. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ. Res. 94, 1507–1514 (2004).
Volonte, D., McTiernan, C.F., Drab, M., Kasper, M. & Galbiati, F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am. J. Physiol. Heart Circ. Physiol. 294, H392–H401 (2008).
Zhao, Y.Y. et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc. Natl. Acad. Sci. USA 99, 11375–11380 (2002).
Lin, J. et al. The regulation of the cardiac potassium channel (HERG) by caveolin-1. Biochem. Cell Biol. 86, 405–415 (2008).
Sinner, M.F. et al. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG). Eur. Heart J. 29, 907–914 (2008).
Zhang, Q. et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 16, 2816–2833 (2007).
Matsuura, T. et al. Two newly identified genomic mutations in a Japanese female patient with fructose-1,6-bisphosphatase (FBPase) deficiency. Mol. Genet. Metab. 76, 207–210 (2002).
Schulze-Bahr, E. et al. Pacemaker channel dysfunction in a patient with sinus node disease. J. Clin. Invest. 111, 1537–1545 (2003).
Milanesi, R., Baruscotti, M., Gnecchi-Ruscone, T. & DiFrancesco, D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N. Engl. J. Med. 354, 151–157 (2006).
Frey, N. & Olson, E.N. Calsarcin-3, a novel skeletal muscle–specific member of the calsarcin family, interacts with multiple Z-disc proteins. J. Biol. Chem. 277, 13998–14004 (2002).
Beqqali, A. et al. CHAP is a newly identified Z-disc protein essential for heart and skeletal muscle function. J. Cell Sci. 123, 1141–1150 (2010).
Arola, A.M. et al. Mutations in PDLIM3 and MYOZ1 encoding myocyte Z line proteins are infrequently found in idiopathic dilated cardiomyopathy. Mol. Genet. Metab. 90, 435–440 (2007).
Brugada, R. et al. Identification of a genetic locus for familial atrial fibrillation. N. Engl. J. Med. 336, 905–911 (1997).
Lubitz, S.A. et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation 122, 976–984 (2010).
Kääb, S. et al. Large scale replication and meta-analysis of variants on chromosome 4q25 associated with atrial fibrillation. Eur. Heart J. 30, 813–819 (2009).
Stranger, B.E. et al. Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 (2007).
Vasan, R.S. et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. J. Am. Med. Assoc. 302, 168–178 (2009).
Johnson, A.D. et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24, 2938–2939 (2008).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
de Bakker, P.I. et al. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum. Mol. Genet. 17, R122–R128 (2008).
International HapMap Consortium. The International HapMap Project. Nature 426, 789–796 (2003).
Acknowledgements
Acknowledgments are contained in the Supplementary Note.
Author information
Authors and Affiliations
Contributions
Study concept and design was determined by P.T.E., K.L.L., C.M.A., B.P.K., M.K.C., J.D., H.V., A.H., Y.N., D.M.R., A.G.U., A.B.N., Y.L., T. Tanaka, B.H.C.S., S.B.F., D.D., S.R.H., E.J.B., V.G. and S. Kääb. Acquisition of data was performed by P.T.E., C.M.A., N.L.G., J.C.B., M.K.C., M.D., J.D.R., A.P., M.F.S., J.D., N.L.S., J.D.S., R.W., J.I.R., L.J.L., T.B.H., U.V., A.H., E.Z.S., M.K., U.B.T., D.C., T.F., R.M., B.M.P., T.M., S.P., H.-E.W., J.C.M.W., A.G.U., F.R., M.S., A.B.N., Y.L., M.H.G., B.H.C.S., A.A., S.R.H., E.J.B., V.G., B.P., H.L., B.F.V., M.B., W.H.L.K. and S. Kääb. Analysis and interpretation of data were performed by P.T.E., K.L.L., N.L.G., M.D.R., A.V.S., D.E.A., M.M.-N., B.P.K., S.A.L., J.C.B., M.D., K.O., J.D.R., J.G.S., M.F.S., K.L., J.D., N.L.S., K.M.R., J.I.R., R.W.D., U.V., G.L., T. Tsunoda, N.S., N.K., B.M.P., S. Kathiresan, D.M.R., B.M., Y.L., M.H.G., D.D., S.R.H., H.L., S.X. and E.J.B. Drafting of the manuscript was carried out by P.T.E., K.L.L., B.P.K., S.A.L. and S. Kääb. Critical revision of the manuscript for important intellectual content was carried out by C.M.A., N.L.G., A.V.S., D.E.A., B.P.K., J.C.B., M.K.C., M.D., J.G.S., A.P., M.F.S., J.D., N.L.S., J.D.S., M.R., K.M.R., D.R.V.W., J.W.M., R.W., J.I.R., L.J.L., T.B.H., U.V., H.V., D.J.M., A.H., L.Y.C., E.Z.S., G.L., U.B.T., P.M.R., D.C., N.S., S.M., K.L.F., B.M.P., T.M., S.P., J.C.M.W., D.M.R., A.G.U., F.R., B.M., M.S., A.B.N., Y.L., M.H.G., O.M., B.H.C.S., S.B.F., A.A., D.D., D.I.C., S.R.H., E.J.B., G.S., S.C., A.C., D.L., J.R. and V.G. Statistical analysis was performed by K.L.L., N.L.G., M.D.R., A.V.S., D.E.A., M.M.-N., B.P.K., J.C.B., J.G.S., M.F.S., K.L., J.D., K.M.R., R.W.D., U.V., G.L., L.M.R., Y.L. and D.I.C. Funding was obtained by P.T.E., C.M.A., M.K.C., J.G.S., N.L.S., J.D.S., J.I.R., L.J.L., T.B.H., H.V., A.H., E.B., U.B.T., P.M.R., B.M.P., T.M., H.-E.W., J.C.M.W., D.M.R., A.G.U., Y.L., O.M., B.H.C.S., S.B.F., A.A., D.D., S.R.H., E.J.B., V.G. and S. Kääb. Study supervision was performed by P.T.E., K.L.L., C.M.A., M.K.C., N.L.S., J.I.R., A.H., P.M.R., B.M.P., J.C.M.W., A.G.U., Y.L., B.H.C.S., S.B.F., S.R.H., E.J.B., V.G. and S. Kääb., P.T.E., K.L.L., C.M.A., N.L.G., M.D.R., A.V.S., D.E.A., M.M.-N., B.P.K., J.C.B., M.K.C., M.D., K.O., T. Tanaka, B.H.C.S., S.B.F., A.A., D.D., J.B., D.I.C., S.R.H., V.G., E.J.B. and S. Kääb had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–3 and Supplementary Note (PDF 803 kb)
Rights and permissions
About this article
Cite this article
Ellinor, P., Lunetta, K., Albert, C. et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44, 670–675 (2012). https://doi.org/10.1038/ng.2261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2261
This article is cited by
-
Stem cell models of inherited arrhythmias
Nature Cardiovascular Research (2024)
-
Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility
Human Genomics (2023)
-
Genetics of atrial fibrillation—an update of recent findings
Molecular Biology Reports (2022)
-
Comprehensive analysis of autophagy-related genes and patterns of immune cell infiltration in valvular atrial fibrillation
BMC Cardiovascular Disorders (2021)
-
Atrial fibrillation—a complex polygenetic disease
European Journal of Human Genetics (2021)