Abstract
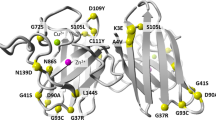
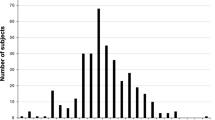
Recent reports have shown heterozygosity for some twenty different mutations in the CuZn–superoxide dismutase (CuZn–SOD) gene in familial amyotrophic lateral sclerosis (FALS), and analysed samples from patients have shown decreased enzymic activity. Here we report homozygosity for an exon 4 mutation, Asp90Ala in fourteen patients among four unrelated ALS families and four apparently sporadic ALS patients from Sweden and Finland. The erythrocyte CuZn–SOD activity is essentially normal. Our findings suggest that this CuZn–SOD mutation causes ALS by a gain of function rather than by loss, and that the Asp90Ala mutation is less detrimental than previously reported mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tandan, R. & Bradley, W.G. Amyotrophic lateral sclerosis Part 1. Clinical features, pathology, and ethical issues in management. Ann. Neurol. 18, 271–280 (1985).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Ogasawara, M. et al. Mild ALS in Japan associated with novel SOD mutation. Nature Genet 5, 323–324 (1993).
Rainero, I. et al. SOD1 missense mutation in an Italian family with ALS. Neurology 44, 347–349 (1994).
Elshafey, A., Lanyon, W.O. & Conner, J.M. Identification of a new missense point mutation in exon 4 of the Cu/Zn superoxide dismutase gene in a family with amyotrophic lateral sclerosis. Hum. molec. Genet. 3, 363–364 (1994).
Jones, C.T., Swingler, R.J. & Brock, D.J.H. Identification of a novel SOD1 mutation in an apparently sporadic amyotrophic lateral sclerosis patient and the detection of lle113Thr in three others. Hum. molec. Genet. 3, 649–650 (1994).
Nakano, R. et al. A novel mutation in Cu/Zn superoxide dismutase gene in Japanese familial amyotrophic lateral sclerosis. Biochem. Biophys. Res. Comms. 200, 695–703 (1994).
Siddique, T., Deng, H.-X., Hentati, A., Hung, W.-Y., He, X.-X. & Mitsumoto, H. Identification of new mutations in famlial amyotrophic lateral sclerosis. Am. J. hum. Genet. 55, A1419 (1994).
Tsuda, T. et al. Analysis of the functional effects of a mutation in SOD1 associated with familial amyotrophic lateral sclerosis. Neuron 13, 727–736 (1994).
Pramatarova, A. et al. Identification of new mutations in the CuZn superoxide dismutase gene of patients with familial amyotrophic lateral sclerosis. Am. J. hum. Genet. 56, 592–596 (1995).
Deng, H.-X. et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261, 1047–1051 (1993).
Robberecht, W. et al. Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 62, 384–387 (1994).
Bowling, A.C., Schulz, J.B., Brown, R.H. & Beal, M.F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 61, 2322–2325 (1993).
Borchelt, D.R. et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. natn. Acad. Sci. U.S.A. 91, 8292–8296 (1994).
Weisiger, R.A. & Fridovich, I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localisation. J. biol. Chem. 248, 4793–4796 (1973).
Marklund, S.L. Human copper-containing superoxide dismutase of high molecular weight. Proc. nafn. Acad. Sci. U.S.A. 79, 7634–7638 (1982).
Gumey, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
McCord, J.M. & Fridovich, I. Superoxide dismutase, an enzymic function for erythrocuprein. J. biol. Chem. 244, 6049–6055 (1969).
Levanon, D. et al. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 4, 77–84 (1985).
Ohman, M. & Marklund, S.L. Plasma extracellular superoxide dismutase and erythrocyte Cu Zn-containing superoxide dismutase in alcoholics treated with disulfiram. Clin. Sci. 70, 365–369 (1986).
Kurland, L.T. & Mulder, D.W. Epidemiological investigations of amyotrophic lateral sclerosis: 2 Familial aggregations indicative of dominant inheritance. Neurology 5, 182–196 & 249–268 (1955).
Chio, A., Brignolio, F., Meineri, P. & Schiffer, D. Phenotypic and genotypic heterogeneity of dominantly inherited amyotrophic lateral sclerosis. Acta Neurol. Scand. 75, 277–282 (1987).
Myllyla, V.V., Toivakka, E., Ala-Hurula, V., Hokkanen, E. & Emeryk-Szajewska, B. Juvenile amyotrophic lateral sclerosis: a report of two cases in a single family. Acta Neurol. Scand. 69, 170–177 (1979).
Hentati, A. et al. Linkage of recessive familial amyotrophic lateral sclerosis to chromosome 2q33–q35. Nature Genet. 7, 425–428 (1994).
Dumon, J., Macken, J. & de Barsy, T.H. Concordance for amyotrophic lateral sclerosis in a pair of dizygous twins of consanguineous parents. J. med. Genet. 8, 113–116 (1971).
Beckman, G. Population studies in northern Sweden VI. Polymorphism of superoxide dismutase. Hereoditas 73, 305–309 (1973).
Beckman, G. & Pakarinen, A. Superoxide dismutase, a population study. Hum. Hereof. 23, 346–351 (1973).
Eriksson, A.W. Genetic polymorphisms in finno-ugrian populations. Israel J. med. Sci. 9, 1156–1170 (1973).
Beckman, G., Beckman, L. & Nilsson, L.-O. Genetics of human superoxide dismutase. Heriditas 79, 43–46 (1975).
Själander, A. et al. Molecular characterisation of a missense mutation in the CuZn superoxide dismutase with normal enzyme activity. Hum. molec. Genet. (in the press).
Forsgren, L., Almay, B.G., Holmgren, G. & Wall, S. Epidemiology of motor neuron disease in northern Sweden. Acta Neurol. Scand. 68, 20–29 (1983).
Tainer, J.A., Getzoff, E.D., Beem, K.M., Richardson, J.S. & Richardson, D.L. Determination and analysis of the 2A structure of copper zinc superoxide dismutase. J. molec. Biol. 160, 181–217 (1982).
Bordo, D., Djinovic, K. & Bolognesi, M. Conserved patterns in the CuZn superoxide dismutase family. J. molec. Biol. 238, 366–386 (1994).
Beckman, J.S., Larson, M., Smith, C.D. & Koppenol, W.H. ALS, SOD and peroxynitrite. Nature 364, 584 (1993).
Yim, M.B., Chock, P.B. & Stadtman, E.R. Enzyme function of copper. zinc superoxide dismutase as a free radical generator. J. biol. Chem. 268, 4099–4105 (1993).
Swash, M. & Leigh, P.M. Criteria for diagnosis of familial amyotrophic lateral sclerosis. Neuromusc. Disord. 2, 7–9 (1992).
Marklund, S.L. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. biol. Chem. 251, 7504–7507 (1976).
Marklund, S.L. Direct assay of superoxide dismutase with potassium superoxide. In Handbook of methods for oxygen radical research. (ed. Greenwald, R.A.) (CRC Press Inc., Boca Raton, 249–255 1985).
Miettinen, O.S. Simple interval estimation of risk ratio. Am. J. Epidemiol. 100, 515–516 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andersen, P., Nilsson, P., Ala-Hurula, V. et al. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet 10, 61–66 (1995). https://doi.org/10.1038/ng0595-61
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0595-61
This article is cited by
-
Widespread CNS pathology in amyotrophic lateral sclerosis homozygous for the D90A SOD1 mutation
Acta Neuropathologica (2023)
-
Therapy development for spinal muscular atrophy: perspectives for muscular dystrophies and neurodegenerative disorders
Neurological Research and Practice (2022)
-
SOD1 D91A variant in the southernmost tip of Europe: a heterozygous ALS patient resident on the island of Gozo
European Journal of Human Genetics (2022)
-
Approaches to Gene Modulation Therapy for ALS
Neurotherapeutics (2022)
-
The Cysteine (Cys) Residues Cys-6 and Cys-111 in Mutant Superoxide Dismutase 1 (SOD1) A4V Are Required for Induction of Endoplasmic Reticulum Stress in Amyotrophic Lateral Sclerosis
Journal of Molecular Neuroscience (2020)