Abstract
Today, there is accumulating evidence from animal experiments that axonal regeneration and an enhanced level of functional repair can be induced after a spinal cord injury (SCI). Consequently, in the near future, new therapeutic approaches will be developed for the treatment of patients with SCI. The aim of the project presented here is to provide the required clinical basis for the implementation of novel interventional therapies. Refined and combined clinical and neurophysiological measures are needed for a precise qualitative and quantitative assessment of spinal cord function in patients with SCI at an early stage. This represents a basic requirement to recognise any improvement in the recovery of function and to monitor any significant effect of a new treatment. To this aim, five European Spinal Cord Injury Centres involved in the rehabilitation of acute SCI patients have built up a close clinical collaboration to develop a standardised protocol for the assessment of the outcome after SCI and the extent of recovery achieved by actually applied therapies in a larger population of SCI patients. The project's aim is to establish objective, refined tools as a basis for monitoring the effects of new treatment strategies.
Similar content being viewed by others
Introduction
Recovery of lost function after spinal cord injury (SCI) is in an exciting phase of research. Recovery after SCI is a multidimensional process that includes different mechanisms (Figure 1). In a clinical rehabilitation setting, recovery of sensorimotor functions is mainly achieved by ‘adaptation’ (eg application of an orthosis) and ‘compensation’ (eg training of new muscle synergies). The physical training is primarily directed to strengthen and optimise the preserved sensorimotor functions. The physical therapy aims to improve the function of both undamaged and, as far as possible, damaged neuronal structures. However, the ‘reorganisation’ of neuronal circuits is also the target of specific training approaches. Especially repetitive training sessions aim to optimise the afferent inputs and to improve complex upper and lower limb movements. In addition, animal experiments currently prepare the basis for future novel therapeutic approaches, including induction of axonal regeneration and plasticity, myelin repair and improved neuroprotective strategies.1
Since the Second World War, epidemiological changes in SCI took place. In the last few decades, especially more frequently incomplete SCIs are transferred sooner to specialised Spinal Cord Injury Centres.2 There is a higher frequency of sports and recreational accidents, and the annual incidence in developed countries varies greatly from 2.1 to 123.6 per million people.3,4,5,6,7,8
In a large outcome study of patients who had sustained a SCI, 37% could not walk, 24% could walk, but used wheelchair for daily locomotion, and 39% were functional walkers.9 Although neurological recovery is influenced by aetiology and severity of injury, there is still no evidence that surgery compared to a conservative treatment enables a favourable outcome.10,11 However, it could be shown that, by clinical examinations, suitable patients for future new pharmacological or surgical treatment might be identified.12 Based on these data as well as the long-term psychosocial effects of SCI,13 recovery of locomotor function is becoming an important goal for an increasing proportion of patients.
Up to now, most rehabilitation approaches have focused on the exploitation of spinal cord plasticity below the level of lesion, for example, by the locomotor training14,15 (for a review, see Dietz16). In the future, it might become feasible to partially repair a spinal cord lesion, for example, by inducing regeneration17 (for a review, see Schwab1, Schwab and Bartholdi18). The effects of these new approaches observed in rat experiments appear to be potentially beneficial with respect to an improved outcome of function after SCI.19 ‘Translating these experimental therapies to human patients is enormously challenging and will involve well integrated groups of basic and clinical scientists working together to cross almost entirely uncharted territory.20 Consequently, clinical centres dealing with spinal cord-injured patients should become prepared to include such novel approaches in their treatment procedures. This means that they should be able to carefully monitor any regeneration effects by clinical, electrophysiological, imaging and behavioural examinations.
The aim of this paper is to indicate the requirement of a comprehensive protocol for the assessment of the course of a SCI, rather than providing a detailed protocol of all possible clinical and neurophysiological examinations. The most important aspect of the ‘comprehensive protocol’ is the combination of clinical (ASIA standards), functional (ADL scores, walking capacity) and neurophysiological assessments. As recovery in neurological disorders is always a complex and multidimensional process, the application of only one of these assessments is not sufficient to estimate and/or evaluate the clinical significance of any new treatment.
New treatment approaches
Approaches using plasticity
Functional recovery after CNS injury depend, in part, upon reorganisation of undamaged neural pathways.21 Spinal cord circuits are capable of significant reorganisation induced by both activity-dependent and injury-induced plasticity. This plasticity becomes obvious in the ability of spinalised animals to regain a certain degree of motor function. Recent work with spinal-injured humans demonstrates that training can greatly improve the functional locomotor abilities (for a review, see Dietz16). New methodologies to enhance limb movement are designed to further exploit the plastic capabilities of the spinal cord by reinforcing appropriate connections in an activity-dependent manner.16,22
It is likely that even the best future therapeutic interventions will not result in complete re-establishment of all the descending and ascending projections within the spinal cord. Thus, it is important to maximise the functional contributions of the limited number of projections that can be encouraged to regenerate. The regeneration of supraspinal axons must establish stable synapses with ‘appropriate’ target neurones in the spinal cord, in order to contribute to functional recovery (for a review, see Schwab,1 Schwab and Bartholdi,18 Raineteau and Schwab21).
Activity-dependent processes play a significant role in appropriate remodelling in CNS pathways including the motor system.21,22 Thus, it is feasible that provision of appropriate patterns of activity within spinal cord circuits will assist in establishing functional connectivity between regenerating supraspinal axons and spinal neurons. Locomotor training, in combination with the appropriate physiological and pharmacological stimulation, can provide appropriate neural activity within spinal locomotor circuits.22 It has therefore a potential to modify and refine the pattern of regenerating synaptic connections in an activity-dependent manner. Thus, the methods described for enhancing locomotor recovery after spinal injury in the absence of axonal regeneration may also play an important role in optimising the functional contribution of regenerating spinal pathways.
Approaches leading to regeneration
There is convincing evidence from animal, namely rat, experiments that regeneration can be induced after a SCI17,23 (for a review, see Schwab,1 Schwab and Bartholdi,18 Fawcett20). Recently, electrophysiological, behavioural, and imaging studies have indicated that the rat represents a reasonable model related to human SCI.24 Consequently, in the near future, new therapeutic approaches to induce some axonal regeneration as well as enhancement of compensatory nerve fibre growth may become available for the treatment of patients with SCI.
Potential therapeutic interventions include application of neuronal survival and growth-promoting factors, ‘bridging’ transplants of foetal spinal tissue, Schwann or olfactory ensheating cells or stem cells25,26 and the neutralisation of myelin-associated factors that inhibit axonal outgrowth.1,17,18,19,24,26 In addition, stimulating regeneration of damaged axons, several of these therapies, such as neurotrophin application26,27 or neutralisation of myelin-associated factors,1,17 also have the potential to facilitate plasticity within intact neural pathways.
With regard to locomotor recovery, transplantation of foetal cells after spinal transection in the rat resulted in significantly improved outcome.28,29 One possible explanation for the enhanced locomotor performance is that the spinal locomotor circuitry can be activated by tonic activation arising from the transplanted tissue, for example, from 5-HT or Dopamine fibres.30 Thus, there are several promising approaches, although still on an experimental level, which indicate that it could be possible in the near future to improve the mobility also of severely affected patients with SCI.
New interventional therapies: requirements for a future clinical application
In 2001, a clinical initiative was started, which aimed to provide the required clinical basis for the implementation of novel interventional therapies on a large scale. To this aim, five SCI Centres involved in the rehabilitation of acute SCI patients (see the participating centres and responsible principal investigators listed at the end of the report), an engineering section of neuroradiology and a basic neurobiologic lab working in spinal tract regeneration, decided to build up a close collaboration, financially supported by the International Institute for Research in Paraplegia (IRP, Zürich). The same standardised assessment protocol for monitoring the extent and characteristics of recovery in SCI will be applied. About 120 acute SCI patients per year will enter this study and will be followed by a standardised evaluation program.
The paper does not intend to give a methodological overview about the test quality of each of the proposed tests. All of them have their own characteristics (dis/-advantages) concerning their specificity and sensitivity (see references).
Clinical outcome measures
The neurological deficit caused by SCI is presently assessed by the internationally accepted and standardised neurological examination protocol of the American Spinal Injury Association (ASIA).31 This assessment represents a semiquantitative tool to monitor motor and sensory neurological deficits. This protocol allows to determine the level of the lesion, to estimate the extent of neurological deficits, and also to predict to some extent the outcome of SCI. The ASIA score allows to describe structural deficits only indirectly, extrapolated from the neurological deficit.
The assessment of the functional outcome is most important to adequately estimate the patient's quality of life. Besides the neurological deficit, the assessments of functional impairment, for example, walking capacity,32,33 (for a review, see Barbeau and Rossignol34) hand function and bladder control,35 provide essential information about the capacity of the upper and lower limbs, as well as autonomic nerve function. The gain in function achieved during the rehabilitation and by specific therapeutic approaches should be documented by specific behavioural tests. Some actual therapeutical approaches, such as the locomotor training,13,14 have greater effects on the walking ability than on the ASIA motor score,15 underlining the requirement for reliable functional tests in addition to the ASIA score, (for a review, see Dietz et al). The walking index for SCI (WISCI) is applied for the evaluation of locomotion36 and the spinal cord independence measure (SCIM) as a standardised rating scale of activities of daily life (ADL).37 Therefore, the SCIM and the WISCI scores aim to assess the functional impairment of a task and not the neurogenic deficit.
For the assessment of walking function, the WISCI walking index was recently described.36 This walking scale has a good validity and reliability, and includes 20 items to assess the walking ability of a patient with incomplete SCI. As the WISCI scale does not really reflect all aspects of locomotor ability in patients with SCI, additional tests (timed up and go, walking distance in 6 min, time needed for a distance of 10 m) have to be implemented. These tests allow to quantitatively assess the walking capacity. Furthermore, they give additional information about the capacity achieved between the levels of the WISCI scale (eg increase in walking speed while requiring the same walking aids).
The SCIM rating scale estimates the impairment in ADL focused on four most important aspects: self-care, respiration, sphincter management and mobility. It provides a total SCIM score of 100 points, while all the subitems (eg feeding, bathing) are weighted (scores from 0 to 15) dependent on their relevance. Therefore, the SCIM score allows to describe the extent of impairment and the necessary support the patient needs to cover daily life activities.
Neurophysiological recordings
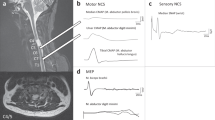
Neurophysiological recordings (neurography, somato-sensory-evoked potentials (SSEP), motor-evoked potentials (MEP)) allow the assessment of ascending and descending spinal tract function. These recordings are not influenced by the cooperation of the patient and can be performed even in unconscious patients (eg due to drugs or in the intensive care unit) or in spinal shock. During the last few years, it could be demonstrated that, by the combination of clinical and neurophysiological recordings (neurography, SSEP, MEP, sympathetic skin response), the functional outcome of patients with an SCI can be predicted with a high reliability within the first 3 weeks after the trauma.38,39 This enables to plan adequate rehabilitation aims and to initiate specific therapeutic approaches (eg functional electrical stimulation) at an early stage (for a review, see Curt and Dietz40). Neurophysiology can be complemented by high-resolution MRI of the spinal cord to provide information about the anatomically preserved parts of spinal cord tracts and consequently of outcome.24,41
Especially, by the combination of MEP, SSEP and neurographic recordings, a highly reliable prediction of the outcome of hand38,39,42 and of locomotor function43,44 can be achieved (for a review, see Curt and Dietz,40 Taylor et al45). The neurographic recordings have been shown to be important in order to assess the extent of intramedullary and peripheral nerve lesions, which represent an essential basis for the planning of rehabilitation approaches.46,47 The neurographic recordings within 10–14 days after trauma indicate if the development of a spastic or flaccid paralysis should be expected. This, for example, determines whether functional electrical stimulation (FES) could be applied, as FES technique is only applicable if there is only a minor damage in the peripheral part of the motor pathways.
Specific goals
The specific aims of our clinical initiative are to develop a comprehensive standard protocol for: (1) early diagnosis, (2) monitoring of the course and (3) prediction of the outcome in acute SCI patients. The combination of the assessment tools and the appropriate timing of the follow-up examinations (from very acute to chronic) allow to monitor the course of the spontaneous recovery, as well as rehabilitation-related gain in function. The proposed protocol is not directed for a specific scientific approach (for example, a new surgical treatment). As all the possible statistical analytical approaches are strongly influenced by the specific intervention, power calculations have to be determined specifically for the actual approach.
First-year experience
Establishment of standardised diagnostic tests
The clinical examination performed according to the ASIA protocol is routine in all centres. It represents one main basis for the prospective studies of the relationship between neurophysiological recordings and clinical outcome.
In addition, a defined set of basic neurophysiological recordings was established in all centres. As neurophysiological recordings were already routinely performed in Garches and Zurich, the techniques established there were taken as a basis for the routine neurophysiological recordings in all the participating centres.
The clinical examinations are paralleled by neurophysiological recordings in every acute patient with a traumatic SCI. The timing of the examinations is scheduled in relation to the date of injury. The very acute examinations are aimed to be performed 5–10 days after injury, the last chronic examinations about 1 year after acute SCI. It could have been shown earlier40 that, by such a time table and sequence of testing, the outcome of the function can be best predicted and monitored over time. It is not intended to acquire data within the first hours after trauma, as such early recordings are less reliable. The time window at 5–10 days also allows a distinction between resolving neuronal conduction blocks (early functional recovery) and postlesion repair mechanisms (weeks to months after injury).
Establishment of functional tests
The following standardised tests for the functional outcome are now established in the five centres. For the walking function, the WISCI walking index, and for hand function, the Sollermann test, and some part of hand function are also reflected in the SCIM score. The Sollermann test was originally suggested to be applied in association with hand surgery. Therefore, the occupational therapists of all participating centres still evaluate reliable tests for hand function that would be most appropriate for tetraplegic patients.
Therapeutical approaches
By the standardised clinical, neurophysiological and functional examinations described above, the course of spontaneous recovery can be monitored under the conventional physical and pharmacological therapies. These standard therapies differ to some extent in each centre. For example, some centres rely more on a Bobath or on a Vojta physical therapy. Furthermore, some centres apply earlier and different antispastic drugs than other ones. It is difficult to completely standardise the routine therapies for all centres. However, these differences in therapy will not affect the clinical and neurophysiological examinations, and the development of a standardised protocol for diagnosis and outcome. The effect of possible differences will be analysed a posteriori.
Coordination of the project
A common database was established for all the participating centres. All clinical and neurophysiological data of each patient who entered one of the five Paraplegic Centres for acute rehabilitation are registered first in the local databases of each centre. By email, the anonymous data sets of each patient are then forwarded to the central database located in Zürich. The data of each patient who gave his/her informed consent are stored in this data bank for comparison and further evaluation.
Consultative and technical meetings are held about every 6 months. Especially, the meetings of occupational and physiotherapists were of great importance to select the same standards for the functional assessments. One of the leaders of the project (AC) visited all the centres to ensure the harmonisation of the technical procedures and to secure the same standard of neurophysiological examinations in each centre. After 1 year of experience with the project, all the centres collaborate in an optimal, well co-ordinated way. Not only was a significant progress of the project achieved, but also the centres themselves profited from the clinical initiative, due to the standardised assessment of patients with SCI.
Next steps to be done
In the next part of the project, new neurophysiological examinations will be introduced, in particular to assess the function of the vegetative system. The sympathetic skin reflexes (SSR) are up to now not routinely recorded in patients with SCI. By this approach, the vegetative system, which is also impaired in most patients with SCI, can be assessed reliably.48,49 The technique is relatively simple, but requires some experience to reliably perform systematic recordings of skin reflexes at different body sites in patients with different levels of SCI. For the assessment of the spinal-segmental pathway, neurographic and electromyographic parameters as well as reflexes will be analysed.
Furthermore, the technology of transcranial magnetic brain stimulation will be established to assess the cortico-spinal tract function. Although it is not yet routinely performed in paraplegic centres, it is clear that double or triple impulses can be applied to the brain to reliably assess the remaining impulse conductivity of the cortico-spinal tract fibres after SCI,50 better than that achieved by single impulse stimulation.
An important aspect in the next years will also be the question, in how far the specific repetitive training of functional movements (hand function, locomotor ability) is able to improve the outcome as compared with the conventional physical therapies.
The present project follows the outline of the WHO for the rehabilitation of SCI patients, which aims to improve the assessment of ‘body structure’ and of ‘body function’. This assessment can serve as the basis to select the best therapeutical approaches to improve activities and participation. In the future, this will be reflected in a better ‘participation’ of these patients in their social surrounding, and also to recognise the relevant environmental factors (for a review, see homepage WHO http://www3.who.int/icf).
References
Schwab ME . Repairing the injured spinal cord. Science 2002; 295: 1029–1031 (review).
Waters RL, Meyer PR, Adkins RH, Felton D . Emergency, acute, and surgical management of spine trauma. Arch Phys Med Rehabil 1999; 80: 1383–1390.
Botterell EH, Jousse AT, Kraus AS, Thompson MG, Wynne-Jones M, Geisler WO . A model for the future care of acute spinal cord injuries. Ann R Coll Phys Surg Can 1975; 8: 193–218.
Gjone R, Nordlie L . Incidence of traumatic paraplegia and tetraplegia in Norway: a statistical survey of the years 1974 and 1975. Paraplegia 1978; 16: 88–93.
Kraus JF . Injury of the head and spinal cord: the epidemiological relevance of the medical literature published from 1960 to 1978. J Neurosurg 1980; 53: 3–10.
Kurtzke JF . Epidemiology of spinal cord injury. Exp Neurol 1975; 48: 163–236.
Minaire P, Castanier M, Girard R, Berard E, Deidier C, Bourret L . Epidemiology of spinal cord injury in the rhone-alpes region, France, 1970–1975. Paraplegia 1978; 16: 76–87.
Shingu H, Ikata T, Katoh S, Akatsu T . Spinal cord injuries in Japan: a nationwide epidemiological survey in 1990. Paraplegia 1994; 32: 3–8.
Burke DC, Burley HAT, Ungar GH . Data of spinal cord injuries: part II, outcome of the treatment of 352 consecutive admissions. Aust NZ J Surg 1985; 55: 377–382.
Marino RJ, Ditunno JF, Donovan WH, Maynard F . Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil 1999; 80: 1391–1396.
Waters RL, Adkins RH, Yakura JS, Sie I . Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord 1996; 34: 188–192.
Burns AS, Lee BS, Ditunno JF, Tessler A . Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma 2003; 20: 477–482.
Bozzacco V . Long-term psychological effects of spinal cord injury. Rehabil Nurs 1993; 18: 82–87.
Dietz V, Colombo G, Jensen L, Baumgartner L . Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 1995; 37: 574–582.
Dietz V, Wirz M, Colombo G, Curt A . Locomotor capacity and recovery of spinal cord function in paraplegic patients. A clinical and electrophysiological evaluation. Electroenceph Clin Neurophysiol 1998; 109: 140–153.
Dietz V . Proprioception and locomotor disorders. Nat Rev Neurosci 2002; 3: 781–790.
Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME . Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 1995; 378: 498–501.
Schwab ME, Bartholdi D . Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996; 76: 319–370.
Thallmair M, Metz GAS, Z'Graggen WJ, Rainteau O, Kartje GL, Schwab ME . Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci 1998; 1: 124–131.
Fawcett J . Repair of spinal cord injuries: where are we, where are we going? Spinal Cord 2002; 40: 615–623.
Raineteau J, Schwab ME . Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2001; 2: 263–273.
Edgerton VR, Roy RR . Paralysis recovery in humans and model systems. Curr Opin Neurobiol 2002; 12: 658–667.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody. Nature 2000; 403: 434–439.
Metz G, Curt A, van de Meent H, Klusman I, Schwab M, Dietz V . Validation of the weight-drop contusion model in rats: a comparative study to human spinal cord injury. J Neurotrauma 2000; 17: 1–17.
Horner PJ, Gage FH . Regenerating the damaged nervous system. Nature 2000; 407: 963–970.
Schnell L, Schneider R, Kollbeck R, Barde YA, Schwab M . Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 1994; 367: 170–173.
Thoenen H . Neurotrophins and neuronal plasticity. Science 1995; 270: 593–598.
Himes BT, Goldberger ME, Tessler A . Grafts of fetal central nervous system tissue rescue axotomized Clarke's nucleus neurons in adult and neonatal operates. J Comp Neurol 1994; 339: 117–131.
Stokes BT, Reier PJ . Fetal grafts alter chronic behavioural outcome after contusion damage to the adult rat spinal cord. Exp Neurol 1992; 116: 1–12.
Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D . Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci 2000; 20: 5144–5152.
Maynard FM, Bracken MB, Creasy G, Ditunno JF, Dunovan WH, Ducker TB . International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997; 35: 266–274.
Waters RL, Yakura JS, Adkins RD . Gait performance after spinal cord injury. Clin Orthop 1992; 14: 87–96.
Yakura J, Waters RL, Adkins RH . Changes in ambulation parameters in SCI individuals following rehabilitation. Paraplegia 1990; 28: 364.
Barbeau H, Rossignol S . Enhancement of locomotor recovery following spinal cord injury. Curr Opin Neurol 1994; 7: 517–552.
Rodic B, Curt A, Dietz V, Schurch B . Bladder neck incompetence in patients with spinal cord injury: significance of sympathetic skin response. J Urol 2000; 163: 1223–1227.
Ditunno Jr JF, Ditunno PL, Graziani V, Scivoletto G, Bernardi M, Casteman V. Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord 2000; 38: 234–243.
Itzkovich M, Tripolski M, Zeilig G, Ring H, Rosentul N, Ronen J et al. Rasch analysis of the Catz-ltzkovich spinal cord independence measure. Spinal Cord 2002; 40: 396–407.
Curt A, Dietz V . Traumatic cervical spinal cord injury: relation between somatosensory evoked potentials, neurological deficit and hand function. Arch Phys Med Rehabil 1996; 77: 48–53.
Curt A, Dietz V . Neurographic assessment of intramedullar motoneurone lesions in cervical spinal cord injury: consequences for hand function. Spinal Cord 1996; 34: 326–332.
Curt A, Dietz V . Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord 1999; 37: 157–165 (review).
Nidecker A, Kocher M, Maeder M, Gratzl V, Zach GA, Benz VF. MR-imaging of chronic spinal cord injury. Association with neurologic function. Neurosurg Rev 1991; 14: 169–179.
Curt A, Keck ME, Dietz V . Functional outcome following spinal cord injury: significance of motor evoked potentials. Arch Phys Med Rehabil 1998; 79: 81–86.
Curt A, Dietz V . Ambulatory capacity in spinal cord injury: significance of somatosensory evoked potentials and ASIA protocols in predicting outcome. Arch Phys Med Rehabil 1997; 78: 39–43.
Li C, Houlden DA, Rowed DW . Somatosensory evoked potentials and neurological grades as predictors of outcome in acute spinal cord injury. J Neurosurg 1990; 72: 600–609.
Taylor S, Ashby P, Verrie M . Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry 1984; 47: 1102–1108.
Krasilowsky G . Nerve conduction studies in patients with cervical spinal cord injuries. Arch Phys Med Rehabil 1980; 61: 204–208.
Rutz S, Dietz V, Curt . Diagnostic and prognostic value of compound motor action potential of lower limbs in acute paraplegic patients. Spinal Cord 2000; 38: 203–210.
Curt A, Weinhardt C, Dietz V . Significance of sympathetic skin response in the assessment of autonomic failure in patients with spinal cord injury. J Auton Nerve Syst 1996; 61: 175–180.
Schurch B, Curt A, Rossier AB . The value of the sympathetic skin responses in the assessment of the vesico-urethral autonomic system. J Urol 1997; 157: 2230–2233.
Abbruzzese G, Assini A, Buccolieri A, Schieppati M, Trompetto C . Comparison of intracortical inhibition and facilitation in distal and proximal arm muscles in humans. J Physiol (Lond) 1999; 514: 895–903.
Author information
Authors and Affiliations
Additional information
Participating Clinical Centres Professor V Dietz, MD; A Curt, MD Spinal Cord Injury Centre University Hospital Balgrist/Switzerland Professor HJ Gerner, MD; R Rupp, dipl.ing. Orthopaedic University Hospital Heidelberg/Germany Professor W Grüninger, MD; M Pott, MD Krankenhaus Hohe Warte Bayreuth/Germany Professor J Duysens, MD Sint Maartenskliniek, Research Department Nijmegen, Netherlands Professor B Bussel, MD Hopital Raymond-Poincarè Paris/France
Participating Basic Science Center Professor M Schwab, PhD Brain Research Institute of the University Zürich Zürich/Switzerland S Kollias, MD Department of Neuroradiology University Hospital Zürich Zürich/Switzerland
Rights and permissions
About this article
Cite this article
Curt, A., Schwab, M. & Dietz, V. Providing the clinical basis for new interventional therapies: refined diagnosis and assessment of recovery after spinal cord injury. Spinal Cord 42, 1–6 (2004). https://doi.org/10.1038/sj.sc.3101558
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101558
Keywords
This article is cited by
-
Promoting FAIR Data Through Community-driven Agile Design: the Open Data Commons for Spinal Cord Injury (odc-sci.org)
Neuroinformatics (2022)
-
Causes of death after spinal cord injury in the Czech Republic
Spinal Cord (2021)
-
Adaptive trial designs for spinal cord injury clinical trials directed to the central nervous system
Spinal Cord (2020)
-
Ulnar nerve integrity predicts 1-year outcome in cervical spinal cord injury
Neurological Research and Practice (2019)
-
Advanced Robotic Therapy Integrated Centers (ARTIC): an international collaboration facilitating the application of rehabilitation technologies
Journal of NeuroEngineering and Rehabilitation (2018)