Article Text
Abstract
Objectives: Downbeat nystagmus (DBN) is the most common form of acquired involuntary ocular oscillation overriding fixation. According to previous studies, the cause of DBN is unsolved in up to 44% of cases. We reviewed 117 patients to establish whether analysis of a large collective and improved diagnostic means would reduce the number of cases with “idiopathic DBN” and thus change the aetiological spectrum.
Methods: The medical records of all patients diagnosed with DBN in our Neurological Dizziness Unit between 1992 and 2006 were reviewed. In the final analysis, only those with documented cranial MRI were included. Their workup comprised a detailed history, standardised neurological, neuro-otological and neuro-ophthalmological examination, and further laboratory tests.
Results: In 62% (n = 72) of patients the aetiology was identified (“secondary DBN”), the most frequent causes being cerebellar degeneration (n = 23) and cerebellar ischaemia (n = 10). In 38% (n = 45), no cause was found (“idiopathic DBN”). A major finding was the high comorbidity of both idiopathic and secondary DBN with bilateral vestibulopathy (36%) and the association with polyneuropathy and cerebellar ataxia even without cerebellar pathology on MRI.
Conclusions: Idiopathic DBN remains common despite improved diagnostic techniques. Our findings allow the classification of “idiopathic DBN” into three subgroups: “pure” DBN (n = 17); “cerebellar” DBN (ie, DBN plus further cerebellar signs in the absence of cerebellar pathology on MRI; n = 6); and a “syndromatic” form of DBN associated with at least two of the following: bilateral vestibulopathy, cerebellar signs and peripheral neuropathy (n = 16). The latter may be caused by multisystem neurodegeneration.
Statistics from Altmetric.com
Downbeat nystagmus (DBN) is the most common form of acquired involuntary ocular oscillations overriding fixation. It is characterised by slow upward drifts and fast downward phases. Slow phase velocity increases on lateral and downward gaze and convergence, although there may be atypical presentations with enhancement of DBN on upward gaze or suppression on convergence.1
The most common presenting symptoms are unsteadiness of gait and to-and-fro vertigo. On further inquiry, patients frequently report blurred vision or oscillopsia that increases on lateral gaze. DBN is often associated with other oculomotor disorders, predominantly smooth pursuit deficits and impairment of the optokinetic reflex and visual fixation suppression of the vestibulo-ocular reflex (VOR).
DBN may be caused by lesions of the vestibulocerebellum and, rarely, bilateral paramedian brainstem pathology.2 In a large proportion of patients, however, no anatomical lesion can be identified (so-called idiopathic DBN).3 The pathophysiology underlying DBN is still controversial. An inherent asymmetry of peripheral vestibular input has been proposed as well as central imbalance in the vertical vestibulo-ocular system.4–6 Others suggest an imbalance of the smooth pursuit system or a mismatch of the coordinate systems of the saccadic burst generator and the neural eye-velocity-to-position integrator.7–9
The aetiology of DBN is diverse. Craniocervical malformations, cerebellar degeneration, vascular pathology, inflammatory disease and intoxication with lithium or antiepileptic drugs have, among others, been implicated.3 5 10–12 In one of the first descriptions of this condition, DBN due to cerebellar ectopia (Arnold–Chiari-malformation (ACM)) was reported.13 In the first survey on DBN, craniocervical malformations were diagnosed in eight of 27 patients and thus topped the list of underlying aetiologies.14 In one of the most comprehensive surveys of this condition to date, these disorders remained one of the most frequent single identified causes of DBN (ACM in 17 of 62 patients).3 In this study, no cause for DBN could be determined in 27 of 62 patients. These retrospective studies, however, were performed before the general availability of MRI. Thus lacunar infarctions, demyelinating plaques and other subtle lesions of the posterior fossa may not have been detected. In a small study of 24 patients with DBN examined by MRI, ACM and cerebellar degeneration remained the commonest causes of DBN.15
To determine if this aetiological spectrum still holds true in an analysis of a large collective with thorough imaging or whether the predominance of craniocervical malformations can be overturned and the number of cryptogenic DBN reduced by improved diagnostic means, we retrospectively analysed the findings in 117 patients who had all undergone cranial MR imaging and standardised neurological, neuro-ophthalmological and neuro-otological examinations.
PATIENTS AND METHODS
To retrospectively determine the frequency of DBN among congenital and acquired ocular oscillations that override fixation, the files of 4854 consecutive patients from our dizziness unit were analysed.
The medical records of 136 patients diagnosed as having DBN in the Neurological Dizziness Unit of the University of Munich over a 14 year period between 1992 and 2006 were reviewed. A detailed standardised history was taken for all patients. The following parameters, all of them relevant to inclusion /exclusion of differential diagnoses of vertigo and dizziness, were assessed.
Vertigo/dizziness/unsteadiness of gait: onset, duration, time course, frequency, associated visual and ocular motor symptoms (including oscillopsia, double vision, blurred vision or loss of sight), associated otological symptoms (including tinnitus, hearing loss, fullness of the ear), associated cerebellar symptoms (ataxia, dysarthria) and other concurrent symptoms, such as headache, phonophobia, photophobia, nausea and vomiting
Past medical history, with particular reference to migraine, polyneuropathy (PNP), exposure to toxins, ethanol, lithium, amiodarone or antiepileptic medication, cardiovascular risk factors (eg, arterial hypertension, diabetes mellitus) and systematic inquiry of other neurological, ophthalmological, ENT and medical diseases
Family history
Patients underwent a full and standardised neurological, neuro-otological and neuro-ophthalmological examination with special emphasis on spontaneous nystagmus with Frenzel’s goggles in the primary position, lateral and vertical gaze direction, gaze evoked nystagmus, smooth pursuit, saccades, optokinetic nystagmus, visual fixation suppression of the VOR, rebound nystagmus, head shaking nystagmus, head thrust test, eye position in roll with a scanning laser ophthalmoscope and determination of the subjective visual vertical.
Electronystagmography
Electronystagmography with bithermal caloric testing (30°C and 44°C) was performed in 71 patients. The mean peak slow phase velocity (SPV) was determined with Igor Pro Wave Metric (V.3.13) software. Vestibulopathy was defined as nystagmus with an SPV <5°/s per irrigation.
Other laboratory tests
Specific laboratory tests included cranial MRI (n = 117), auditory evoked potentials and/or an audiogram (n = 27), vitamin B12 (n = 50), folate (n = 48), vitamin E (n = 24), glutamic acid decarboxylase antibodies (n = 8), magnesium (n = 20) and CSF chemistry (n = 62).
PNP was diagnosed in those patients with electromyographic signs of axonal and/or demyelinating PNP and in those with a significantly reduced sense of vibration (4/8 or less in the tuning fork test).
Statistics
Data were collected and evaluated by means of Excel (Microsoft Corp.) spreadsheet software. Comparison between categorical values was performed using a χ2 test. Metric values were compared by a Mann–Whitney U test. Significance was defined as an alpha level of 0.05.
RESULTS
Of a total of 5904 patients in our department between 1992 and 2006, 136 had been diagnosed as having DBN. DBN was found to be the most common form of involuntary fixation nystagmus of central origin (table 1). Only those patients who had cranial MRI (n = 117) were included in the final analysis. They were evaluated for aetiology, epidemiology, presentation and associated findings.
Aetiology
In 62% (72 of 117) of patients with DBN, a possible (n = 46) or definite (n = 26) causative factor was identified. The most frequent single identifiable cause of DBN in our patients was cerebellar degenerative disease (20%, n = 23). This group included multisystem atrophy (according to the diagnostic criteria of the International Consensus Conference), spinocerebellar ataxia (ataxia with suspected autosomal dominant inheritance) and sporadic adult onset ataxia (progressive ataxia without established symptomatic cause).16 17 In one patient, the diagnosis (spinocerebellar ataxia 6) was confirmed by genetic testing.
Degenerative cerebellar disease as a cause for DBN was followed in frequency by posterior fossa vascular lesions (9%, n = 10), and craniocervical malformations with cerebellar ectopia (7%, n = 8) (fig 1; for a list of individual entities included in the diagnostic groups listed above see tables 2 and 3).
In 38% (n = 45) of all patients with DBN, no causative factor was identified. In these cases DBN was therefore considered idiopathic. As “idiopathic DBN” might represent a distinct nosological entity of unknown aetiology, it will henceforth be considered separately, especially in terms of its association with other neurological symptoms.
Age and gender
Median age of all DBN patients was 62 years (range 10–92). In those with idiopathic DBN, median age was 67 years with a peak between the seventh and eighth decade (range 24–92). Patients with secondary DBN had a mean age of 59 years, with the peak onset in the seventh decade (range 10–82 years) (fig 2). Thus patients with idiopathic DBN were older than those with secondary DBN (p<0.001). Whereas the male-to-female ratio was roughly equal in idiopathic DBN, there was a slight female preponderance in secondary DBN (total male to female: 55% vs 45%; idiopathic: 51% vs. 49%; secondary: 41% vs 59%).
Characteristic features of DBN
A typical feature of DBN is enhancement of the SPV on lateral gaze. Some patients, however, lack enhancement or even exhibit a reduction in SPV. In our series, SPV increased on lateral gaze in 77% (n = 90) of patients and there was no SPV enhancement in 18% (n = 21) of patients (n = 41 vs n = 4 in idiopathic DBN and n = 49 vs n = 17 in secondary DBN). In six patients, modulation of DBN on lateral gaze had been insufficiently documented. Idiopathic DBN was more often associated with enhancement of the nystagmus on lateral gaze than secondary DBN (p<0.05).
In 64% (n = 29) of patients with idiopathic and 82% (n = 59) of those with secondary DBN, SPV increased on downward and decreased on upward gaze. In 6% (n = 3) of patients with idiopathic and none of those with secondary DBN, SPV increased on upward gaze. Convergence enhanced SPV in 64% (n = 29) of patients with idiopathic and 61% (n = 44) of patients with secondary DBN. Dependency of SPV on vertical gaze position and convergence was not statistically different between idiopathic and secondary DBN.
In nine patients (six with idiopathic, three with secondary DBN), dependency of the SPV on head position was tested. In seven (four idiopathic DBN, three secondary DBN) patients, SPV decreased when the patient was supine and increased when he was prone. In one patient with idiopathic DBN, SPV increased in both the supine and prone positions while in another it decreased in both positions.
Presenting symptoms and clinical signs
The most common presenting symptom was unsteadiness of gait or to-and-fro vertigo (idiopathic 89%; secondary 81%). Most patients with idiopathic or secondary DBN reported permanent rather than episodic symptoms (93% vs 94%). Oscillopsia was reported by 44% with idiopathic compared with 38% with secondary DBN.
Associated neurological findings
Peripheral vestibular deficits
A frequent finding in patients with DBN was unilateral or bilateral vestibulopathy. Vestibulopathy was defined as a reduced response to caloric irrigation and/or a pathological head thrust test. Forty-five patients had undergone either caloric irrigation or head thrust tests, 36 had undergone both and in 36 patients (28 with secondary, eight with idiopathic DBN) peripheral vestibular function was not tested. Of those tested, 32% of patients with idiopathic DBN and 39% with secondary DBN had bilateral vestibular deficits. Of those who had undergone both caloric irrigation and head thrust tests (n = 36), 19 had bilateral vestibulopathy. In nine of these, both tests showed bilateral vestibular deficits. Eight had pathological head thrust tests with normal caloric excitability and two patients exhibited diminished peripheral vestibular caloric response with bilateral (n = 1) or unilateral (n = 1) physiological head thrust tests.
Polyneuropathy
PNP was frequently associated with DBN (16 patients with idiopathic, 18 with secondary DBN). Patients with known aetiologies of PNP, such as diabetes mellitus (n = 9), were excluded from this group. The neuropathies diagnosed were heterogeneous, including sensory, motor, demyelinating, axonal and mixed types.
Cerebellar signs
Cerebellar signs, including limb ataxia and dysarthria, were frequently seen in the “secondary DBN” group (limb ataxia in 70% and dysarthria in 46%). They were also present in patients with idiopathic DBN (limb ataxia in 42% and dysarthria in 13%)—that is, those patients who did not have cerebellar lesions on MRI or evidence of cerebellar disease in other technical investigations.
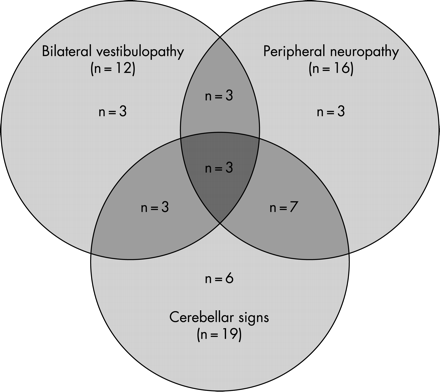
Notably, there was considerable overlap between idiopathic DBN, cerebellar signs, bilateral vestibulopathy and peripheral neuropathy (fig 3). Thirteen patients had DBN associated with varying combinations of two of these disorders and, in three patients, with all three of them.
Associated neuro-ophthalmological findings
The most frequent ophthalmological findings associated with DBN were saccadic vertical and horizontal smooth pursuit (idiopathic DBN 97% and secondary DBN 99%), impaired vertical optokinetic reflex (idiopathic DBN 85%, secondary DBN 87%) and impaired horizontal fixation suppression of the VOR (idiopathic DBN 73%, secondary DBN 70%). Other pathologies included incomplete ocular tilt with deviation of the subjective visual vertical and/or ocular torsion (idiopathic DBN 38%, secondary DBN 41%), dysmetric/slowed saccades (idiopathic DBN 31%, secondary DBN 59%), rebound nystagmus (idiopathic DBN 18%, secondary DBN 29%) and head shaking nystagmus (idiopathic DBN 11%, secondary DBN 16%).
Ancillary findings
Cranial imaging
A cranial MRI was obtained for all 117 patients. An experienced neuroradiologist detected cerebellar atrophy in 27 patients and other cerebellar lesions in 13 patients. Brainstem lesions were present in 11 patients.
Serum chemistry
Vitamin B12 serum levels were determined in 50 patients. Two had markedly diminished levels (146 pg/ml, 95 pg/ml). B12 was only slightly reduced in two others (211 pg/ml, 239 pg/ml; normal range 250–900). Vitamin E levels were determined in 24 patients; all were within the normal range or elevated. Furthermore, magnesium levels were determined in 20 patients, all of which were normal.
Glutamic acid decarboxylase antibody levels were determined in eight patients. Markedly raised serum levels were detected in a 56-year-old male with a history of chronic lymphocytic leukaemia (80.2 U/ml; normal range <0.9).
Spinal fluid
A spinal tap was performed in 62 patients. Six patients had a raised cell count (range 8–229 cells), nine patients raised CSF protein (range 50–150 mg/dl) and six patients had CSF specific oligoclonal bands. The diagnoses in patients with pathological CSF included cerebellitis, pontine encephalitis and paraneoplastic disease.
Drugs
Amiodarone toxicity was the cause of DBN in one patient, whose nystagmus disappeared after discontinuation of the drug. In two of nine patients on anticonvulsive medication at the time of diagnosis, drug toxicity was deemed responsible for the DBN: a 73-year-old female with blood phenytoin levels of 30.3 µg/ml and a 58-year-old female on carbamazepine and vigabatrin.
DISCUSSION
This retrospective study has shown that despite improved diagnostic means, especially the use of high resolution MRI, the percentage of patients in whom no aetiology of DBN could be determined, so-called “idiopathic DBN”, remains high (38%). This parallels values obtained in earlier studies performed before the general availability of MRI and/or with smaller samples (idiopathic DBN in 25–44% of patients).3 14 15 While our study has reaffirmed the preponderance of cerebellar degeneration, posterior fossa vascular lesions and cerebellar ectopia among the causes of secondary DBN, it has also found new aspects connected with the comorbidity, classification and possible pathomechanisms of DBN.
Association of DBN with bilateral vestibulopathy, cerebellar ataxia and polyneuropathy
A major finding of this study was the association of idiopathic as well as secondary DBN with bilateral vestibulopathy (BVP), as demonstrated by head thrust tests or caloric irrigation. The prevalence of DBN and BVP is not known. In our neurological ocular motor and dizziness unit, BVP accounts for 4% and DBN for 2.3% of the diagnoses. The common comorbidity of BVP and DBN (32% in idiopathic and 39% in secondary DBN) suggests a common pathomechanism. This is supported by a recent study on 255 patients with BVP, 17 (7%) of whom also had DBN.18
Apart from BVP, cerebellar ataxia and dysarthria were identified as frequent concurrent symptoms. The association of DBN with cerebellar symptoms is not surprising as DBN is caused by a cerebellar, namely Purkinje cell, dysfunction. Forty-two per cent of patients who fulfilled the criteria of so-called idiopathic DBN had cerebellar symptoms, although usually subtle ones. In this group of patients, MRI detected no cerebellar atrophy or other cerebellar lesions. At this point, however, we cannot exclude the fact that at least a subgroup of these patients presented at an early stage of a cerebellar degenerative disease before overt cerebellar atrophy. Therefore, follow-up-studies are required.
The third clinical entity frequently coexistent with DBN was PNP (36% in idiopathic and 25% in secondary DBN). These numbers may be biased by the advanced age of our sample. In a community based survey, PNP had a prevalence of 19% in a population between the ages of 65 and 74 years who had no disease known to cause PNP.19
Among the group fulfilling the current criteria for idiopathic DBN, a subgroup of 16 patients (36%) were identified in whom DBN was associated with two or all of the following disorders: cerebellar signs, BVP and/or PNP. In a recent study on four patients with cerebellar ataxia and bilateral vestibulopathy, three patients also had sensory neuropathy and one patient DBN.20 In these patients, however, MRI revealed cerebellar atrophy. Thus to the best of our knowledge this is the first sample of patients with the specific association of DBN, BVP, PNP and cerebellar signs without imageable cerebellar atrophy.
Pathomechanisms of DBN
DBN has long been associated with cerebellar lesions, particularly with those of the floccular and parafloccular lobes.8 21–23 Several models of the pathomechanism of DBN have been suggested, among them an imbalance in the smooth pursuit system, or a central or peripheral vestibular imbalance.4–7 24 Recent therapeutic advances with 4-aminopyridine, a potassium channel blocker, support this hypothesis. This agent may enhance the inhibitory output of floccular Purkinje cells on vestibular nuclei and thus alleviate DBN.25
On the basis of our findings that DBN frequently coexists with BVP, we suggest a potential pathophysiology of idiopathic DBN. In our collective, DBN was predominantly associated with high frequency vestibular deficits. This is particularly interesting in the light of recent findings on murine CACNA1A mutants, in which calcium currents through P/Q channels are reduced.26 27 These channels are particularly numerous in cerebellar Purkinje cells. The mutants “tottering” and “rocker” exhibit an upward elevation of average vertical eye position, a finding possibly analogous to DBN in humans. In these mutants, VOR gain is reduced only at high stimulus frequencies. This parallel supports the view that a channelopathy might be the underlying cause in some cases of idiopathic DBN, a hypothesis that is further backed by the finding that 4-aminopyridine not only decreases DBN SPV but also improves horizontal and vertical VOR gain.28
Another interesting feature of the murine mutants described by Stahl and colleagues26 27 is that they exhibit no or little vertical eye displacement at birth. With advancing age, however, the ocular upward elevation increases. DBN, and particularly idiopathic DBN, in humans is a disorder of the elderly (fig 2). Therefore, we suggest that a genetic polymorphism of the CACNA1A gene predisposes to development of DBN, which becomes manifest if additional degenerative changes develop with ageing. Further genetic and molecular studies need to be performed to prove these hypotheses.
Clinical resume
Neurologists should be familiar with DBN as it is the most common form of involuntary central fixation nystagmus. They should always look for DBN in a patient complaining of gait unsteadiness as this is the most common presentation. Particular attention should be paid to associated BVP, cerebellar symptoms and PNP. On the basis of our results, we suggest a subgrouping of “idiopathic DBN”: (1) patients with “pure” DBN who have no other signs or symptoms except for the oculomotor disorders commonly associated with floccular disorders (ie, impaired smooth pursuit, optokinetic reflex and fixation suppression of the VOR); (2) a “cerebellar” form (ie, DBN associated with cerebellar ataxia or dysarthria but without cerebellar atrophy on routine MRI). This form may be a distinct nosological entity or the early manifestation of another cerebellar degenerative disease. Follow-up studies will be needed to clarify this issue; and (3) a “syndromatic” form (ie, DBN associated with at least two of the following: BVP, cerebellar signs and/or PNP). The association of these disorders suggest that this syndrome may reflect a multisystem neurodegeneration or channelopathy.
Acknowledgments
We thank Judy Benson for copyediting the text.
REFERENCES
Footnotes
Competing interests: None.