Article Text
Abstract
Objective To analyse predictors for relapses and number of attacks under different immunotherapies in patients with neuromyelitis optica spectrum disorder (NMOSD).
Design This is a retrospective cohort study conducted in neurology departments at 21 regional and university hospitals in Germany. Eligible participants were patients with aquaporin-4-antibody-positive or aquaporin-4-antibody-negative NMOSD. Main outcome measures were HRs from Cox proportional hazard regression models adjusted for centre effects, important prognostic factors and repeated treatment episodes.
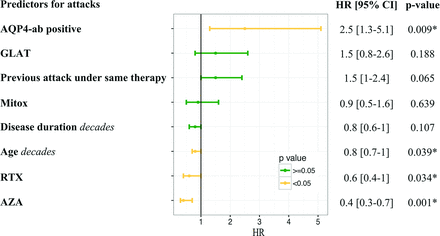
Results 265 treatment episodes with a mean duration of 442 days (total of 321 treatment years) in 144 patients (mean age at first attack: 40.9 years, 82.6% female, 86.1% aquaporin-4-antibody-positive) were analysed. 191 attacks occurred during any of the treatments (annual relapse rate=0.60). The most common treatments were rituximab (n=77, 111 patient-years), azathioprine (n=52, 68 patient-years), interferon-β (n=32, 61 patient-years), mitoxantrone (n=34, 32.1 patient-years) and glatiramer acetate (n=17, 10 patient-years). Azathioprine (HR=0.4, 95% CI 0.3 to 0.7, p=0.001) and rituximab (HR=0.6, 95% CI 0.4 to 1.0, p=0.034) reduced the attack risk compared with interferon-β, whereas mitoxantrone and glatiramer acetate did not. Patients who were aquaporin-4-antibody-positive had a higher risk of attacks (HR=2.5, 95% CI 1.3 to 5.1, p=0.009). Every decade of age was associated with a lower risk for attacks (HR=0.8, 95% CI 0.7 to 1.0, p=0.039). A previous attack under the same treatment tended to be predictive for further attacks (HR=1.5, 95% CI 1.0 to 2.4, p=0.065).
Conclusions Age, antibody status and possibly previous attacks predict further attacks in patients treated for NMOSD. Azathioprine and rituximab are superior to interferon-β.
- Neuromyelitis optica spectrum disorder
- Therapy
- Azathioprine
- Rituximab
- Aquaporin-4 antibody
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune disease of the central nervous system, mainly manifesting through recurrent attacks of optic neuritis and longitudinally extensive transverse myelitis.1 Antibodies to aquaporin-4 (AQP4-abs) were identified as pathogenic, and their detection, in addition to typical clinical manifestation, is a hallmark of the recently updated diagnostic criteria.2–4 The presence of AQP4-abs is also related to prognosis and attack risk.5 6 While a chronic progressive disease course is very rare, inflammatory disease attacks are associated with a high risk of persisting disability, including paraplegia and blindness.7 8 Attack prevention with immunosuppressive drugs is currently considered the best available treatment.9–11 Besides classical immunosuppressant drugs such as azathioprine (AZA) or mycophenolate mofetil (MMF), rituximab (RTX) has increasingly been used for the treatment of patients with NMOSD since 2005.12–17 More recently, an interleukin-6 receptor inhibitor (SA-237), eculizumab and an anti-CD19 antibody (inebilizumab) are being investigated as alternative therapies.18–21 However, currently only low evidence exists concerning comparative treatment efficacy. The best available data so far, generated in a retrospective analysis of 90 patients with NMOSD from the USA22 and of 138 patients with NMOSD from Korea,23 describe greater efficacy of RTX and MMF compared with AZA. Furthermore, little is known about predictors for treatment response,24 and it is unknown whether AQP4-ab serostatus, gender, age, disease duration and other clinical parameters are associated with attack-free survival under treatment. As long as large prospective cohorts or randomised trials, which are difficult to perform due to the rarity of NMOSD, are lacking, retrospective cohort studies are the best available approach to gain further knowledge about treatment efficacy and predictors of treatment response. Using the NMOSD registry of the German Neuromyelitis Optica Study group (NEMOS), we analysed the efficacy of immunotherapies for attack prevention and predictors for attacks under immunotherapies.
Methods
Study design and patients
This retrospective cohort study was based on the German NEMOS group (www.nemos-net.de) registry established in 2008. At database lock, the registry included 186 patients with neuromyelitis optica (NMO) diagnosed according to the 2006 Wingerchuk25 criteria or with AQP4-ab-positive NMO spectrum disorder (NMOSD). The local institutional review boards of the participating centres approved the study (first approval from the institutional review board Charité Universitätsmedizin Berlin EA3/004/08). Last data entry for this analysis varied between centres and was between January 2012 and March 2013.
Data collection and processing
Data collected at regular clinical visits included demographic data, AQP4-ab status, attacks (onset, treatments and outcome), long-term treatments (compounds, start/stop dates and dosages), expanded disability status scale and visual acuity. A detailed description of the cohort and the methods used for data collection, including an on-site data validation (‘flying doctor-approach’), has been published previously.8
For this study, demographic data, long-term treatment data and attack dates were extracted from the database. Treatment data were validated through manual quality checks performed by two authors (JPS and MK), as well as by automated logical checks. As further analyses relied on exact treatment data, including start and stop dates, patients with insufficient baseline or treatment data were excluded.
Definition of treatment episodes
For our analyses which were based on pharmacodynamics and previous treatment experience, we determined the efficacy of therapeutic interventions after the last dose as follows: 365 days for alemtuzumab; 180 days for RTX; 90 days for mitoxantrone (Mitox) and intrathecal steroids; 30 days for cyclophosphamide, AZA, ciclosporin A, MMF, natalizumab, intravenous immunoglobulin, fingolimod (FTY), intravenous steroids and tocilizumab (TCZ); and 7 days for interferon-β (IFN), glatiramer acetate (GLAT), methotrexate and oral steroids. Treatment duration was prolonged if the documented stop date (ie, clinical decision to not further proceed with the current treatment) was before the end of effectiveness as defined above. In recurrent treatment episodes with the same compound (observed for AZA, RTX, Mitox and IFN), we merged the two cycles if the first dose of the second episode was administered less than 30 days (AZA, RTX, Mitox) or 10 days (IFN) after the assumed end of effectiveness, for example two RTX cycles 200 days apart were considered as one continuous treatment episode. For other treatments, no recurrent treatment episodes were observed. Gaps longer than the mentioned period were treated as separated episodes in further analyses. Treatments with uncertain treatment start or stop dates were excluded. Finally, in 59 treatments an overlap of treatment durations as defined by the above-mentioned effectiveness time or a combination of two or three compounds occurred (online supplementary table 1). Unfortunately, these groups were too small and too heterogeneous to reliably investigate combination therapies. Therefore, our analyses were restricted to monotherapies.
Data sets
Two data sets were defined. Data set A included all available treatment data for descriptive statistics, its changes over time and for computing unadjusted annual relapse rates (ARR).
In Data set B, recurrent event analyses considering the time from treatment start to attack were performed. Treatment episodes from patients with an attack under the treatment were split in separate episodes by the date of attack onset. Each treatment episode was labelled as stable if no attack occurred and as failure if an attack occurred. Treatment episodes shorter than 14 days or with unreliable start and stop dates were excluded. For efficacy analyses and response predictors, we reduced the data set to compounds with at least 10 patient-years in at least 10 patients (Data set B).
Statistical analyses
Our statistical analysis plan was designed (1) to investigate prescription reality of immunotherapies in Germany and their changes over time, (2) to compare efficacy of treatments in recurrent event analyses and (3) to explore the data set for predictors of relapses.
Using Data set A, descriptive statistics of the cohort were performed. To investigate if prescription routine changed over time, the χ2 test was used to compare frequencies before and after publication of the German NMO treatment guidelines by NEMOS in early 2011.26 Under the assumption that attack occurrence follows a Poisson distribution, we estimated annualised relapse rates (ARR, mean number of relapses per treatment year) and 95% CIs. ARR estimates were unadjusted for any covariates. Differences between the cohorts were tested with χ2 test (rates) or analysis of variance (continuous data).
For the analysis of Data set B, the multivariate cox proportional hazard regression models for recurrent events were computed.27 We aimed to compare treatment effects and to investigate the influence of potential predictors of attack risk: age, gender, previous attack under therapy, line of treatment for the individual patient (labelled as first line, second line, or third or more line), AQP4-ab status and whether the 2006 Wingerchuk criteria were fulfilled. The patient ID was included as cluster variable to account for intraindividual correlation of observations, and the models were additionally corrected for centre effects. In a first step, a multivariate model was performed, including predictors and treatments, and HRs and their 95% CIs were computed. Second, the number of variables in the model was reduced by excluding all variables not showing at least a trend towards significance (defined as p<0.1). As no untreated or placebo cohort for estimating HRs was available, IFN was chosen as a reference category for all treatment comparisons, as IFN was shown to be without clinical efficacy in NMOSD.28 To compare predictors between different treatments, we performed post-hoc analyses for each treatment subgroup. p Values <0.05 were considered statistically significant. All analyses were performed with Statistics in R (V.3.2.3), including the survival package.29
Results
Description of the cohort
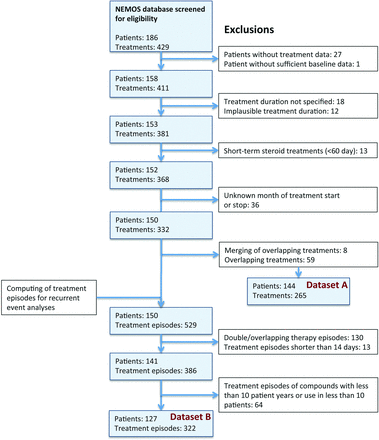
One hundred and eighty-six patients with NMO or AQP4-ab-positive NMOSD were documented in the NEMOS registry. After exclusion of incomplete and uncertain treatment data as well as overlapping treatments, 265 different treatments in 144 patients from 21 centres could be extracted (figure 1). The majority of patients were female (n=119, 82.6%), fulfilled the 2006 Wingerchuk diagnostic criteria (n=113, 78.5%) and were seropositive for AQP4-ab (n=124, 86.1%). The mean age at disease onset was 40.9 (SD: 14.3) years and the median follow-up time was 6.1 (0.1–34.9) years. The median number of immunotherapies per patient was 2 (1–8). The mean duration of treatments was 442 (SD: 432) days, summing up to a total of 321 documented treatment years. One hundred and thirty-seven treatments were given as first-line therapy in the individual patient, 69 as second-line and 59 as third-line (or more). One hundred and ninety-one attacks occurred during the documented treatments, resulting in an overall ARR of 0.60 (95% CI 0.51 to 0.69) in treated patients.
Flow chart of data processing and exclusions. Data set A used for descriptive statistics. Data set B included in recurrent event analyses considering the time to the next attack. For details, see the Methods section. NEMOS, Neuromyelitis Optica Study group.
Frequencies, prescription changes and unadjusted attack rates for all treatments
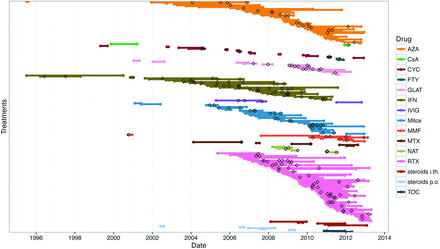
We identified 15 different immunotherapy drugs, which were used in 1–65 (median: 7) patients (table 1). The most commonly used compounds were RTX, with 111 patient years in 65 patients, AZA with 68 patient-years in 46 patients and IFN with 61 patient-years in 30 patients. The mean treatment duration ranged from 77 (FTY) to 699 (IFN) days. Unadjusted ARRs and 95% CI of all treatments are presented in table 1. The prescription of compounds changed over time. Of the most commonly prescribed drugs, RTX (first given in 2005) showed an increase from 24% to 43% of all prescriptions with the cut-off of 2011, whereas Mitox use decreased from 16% to 2% and IFN treatment was not further initiated after 2011. The use of all medications over time and of all relapses occurring during treatment are visualised in figure 2.
Treatments in the Neuromyelitis Optica Study group cohort. Lines represent all treatment episodes over time. Attacks are marked as black diamonds. Data are sorted by compounds. AZA, azathioprine; CsA, ciclosporin A; CYC, cyclophosphamide; FTY, fingolimod; GLAT, glatiramer acetate; IFN, interferon-β; IVIg, intravenous immunoglobulins; Mitox, mitoxantrone; MMF, mycophenolate mofetil; MTX, methotrexate; NAT, natalizumab; RTX, rituximab; i.th., intrathecal; p.o., per os; TCZ, tocilizumab.
Descriptive statistics per treatment (data set A)
Detailed dosing data for these treatments were available in most cases (AZA: 94%, GLAT: 78%, IFN: 88%, Mitox: 87%, RTX: 98%). GLAT patients received 20 mg/day, 69% of IFN treatments used the full dose and 31% a reduced dose (eight times IFN-β-1-a subcutaneous 22 µg, one time IFN-β-1-a intramuscular 8 mg). The median dose of Mitox was 12 mg/m2 body surface (range: 10–12 mg) every 3 months. The median dose of AZA was 150 mg per day (mean: 126 mg, range: 50–300 mg). Fifty per cent of AZA doses were 150 mg or above and 82% at least 100 mg/day. However, sufficiency of AZA dosing could not be reliably evaluated, as body weight data were not available. The median dose of 1000 mg per cycle (one or two infusions) of RTX (range: 375–3000 mg) or more was applied in 79% of treatments, usually every 6 months.
The compounds AZA, IFN and RTX were the most often used first-line therapies in our cohort. RTX was the most often used second-line or third-line therapy. The sequence of compounds used was very heterogeneous, and we did not observe any obvious pattern of typical therapy sequences.
Analysis of predictors of attack risk
Five treatments (AZA, GLAT, IFN, Mitox and RTX) had at least 10 patient-years in at least 10 patients. Three hundred and twenty-two treatment episodes from 127 patients (20 centres) labelled as ‘stable’ (n=191, 59%) or ‘failure’ (n=131, 41%) were included in these analyses. The observation time in this data set included a total of 261.7 patient-years. The five treatment cohorts did not differ significantly concerning gender, proportion of patients positive for AQP4-ab or meeting the 2006 Wingerchuk criteria,25 respectively (table 2).
Descriptive statistics of groups used for survival analyses (data set B)
The analysis for independent treatment response predictors revealed that AQP4-ab-positive patients had a higher risk of attacks than AQP4-ab-negative patients (HR=2.54, 95% CI 1.26 to 5.12, p=0.009). A previous attack under the same treatment tended to increase the risk of a subsequent attack (HR=1.53, 95% CI 0.97 to 2.42, p=0.065). In contrast, every added decade of age was associated with a lower attack risk (HR=0.82, 95% CI 0.69 to 0.99, p=0.039). Other variables were not significantly associated with attack risk (figure 3). Post-hoc, we analysed if predictors differed between the treatments. However, due to the low patient numbers and the lack of events in certain subgroups, we could not reliably estimate HR in every case. For AZA and IFN, neither age, disease duration, previous attacks nor AQP4-abs were predictive for attacks. In contrast, patients treated with RTX had a higher risk for attacks if they were positive for AQP4-abs (HR estimate unreliable, p<0.001). The attack risk under Mitox decreased with every decade of age (HR=0.54, 95% CI 0.35 to 0.85) and was increased after a previous attack under Mitox (HR=2.84, 95% CI 1.17 to 6.88). Decades of age (HR=0.54, 95% CI: 0.37 to 0.79, p<0.001) and disease duration (HR=0.04, 95% CI 0.01 to 0.30) were predictive for fewer attacks under GLAT, while the presence of AQP4-ab was associated with higher attack risk (HR estimate unreliable, p<0.001).
HRs for predictors and treatments. HR and 95% CIs for clinical predictors of treatment response and different treatments (interferon-β as reference). Age and disease duration as decades. Variables not included in the final model: line of therapy, gender and whether the 2006 Wingerchuk criteria were fulfilled. For details, see the Methods section. AQP4-ab, antibodies against aquaporin-4; AZA, azathioprine; GLAT, glatiramer acetate; Mitox, mitoxantrone; RTX, rituximab. *Represent statistically significant data.
Adjusted for AQP4-ab status, age, disease duration and any previous attacks under a therapy, only AZA (HR=0.43, 95% CI 0.26 to 0.71, p=0.001) and RTX (HR=0.60, 95% CI 0.38 to 0.96, p=0.034) reduced the attack risk compared with IFN therapy (figure 3). No statistically significant differences were found between IFN and GLAT or Mitox.
As only AZA and RTX were superior to IFN, we added explorative analyses to elucidate if subgroups of patients might have a more pronounced benefit from either treatment. Within models of direct comparison, neither the use as a first-line nor as second-line/third-line treatment revealed differences between the two drugs. A previous treatment failure under AZA did not predict a better treatment response under RTX when compared with the AZA period in the same patient; however, only 10 patients were available for this analysis. A comparison vice versa was not possible as only three patients were switched from RTX to AZA. Due to the shift towards more RTX prescriptions, the influence of the year of treatment start was additionally tested, but a significant influence was not detected.
Discussion
Patients with NMOSD should receive immunotherapy as early as possible, as any further attack may cause serious and often irreversible disability.10 11 30 However, current knowledge about sequences of immunotherapies, predictors for therapy response or comparison between treatments is limited. The NEMOS database allowed us to analyse ‘real life’ management of NMOSD in a large cohort of 144 patients with NMOSD and an observation period of 321 treatment years.
Patients were cared for at 16 tertiary centres and 5 regional hospitals, therefore covering a study population representative for German patients with NMOSD and the diversity of care. Fifteen different immunotherapeutic drugs were used for the treatment of NMOSD, indicating a heterogeneous treatment of our cohort. The most commonly used drugs were RTX, AZA, IFN, Mitox and GLAT. The high prevalence of drugs regularly used for mulitple sclerosis (MS) and now abandoned for NMOSD probably is due to initial misdiagnosis of patients before AQP4-ab testing has become widely available and the fact that inefficacy of these drugs for NMOSD was unknown at that time. As the data closure of this study was already in 2013, data on longer use of RTX and on some of the recently emerging therapies such as TCZ are limited.
Overall, we found moderate to low unadjusted ARRs under RTX and AZA, the two most commonly used drugs for NMOSD in our cohort. This is in line with other studies reporting ARRs under these therapies of between 0.1 and 0.9.6 12 14 15 17 24 31–33
The proportion of patients treated with drugs regularly used for MS therapy declined over the observation period and RTX gained share, in line with its introduction as first-line recommendation for NMOSD in Germany.26 Of note, while initiation of a drug is guided by recommendations, staying on a drug is influenced by the absence of disease activity and adverse effects. The relative gain of RTX over AZA since 2011 might also be related to its better tolerability.
Using a multivariable model, we compared the effect on disease activity of different immunotherapies. RTX and AZA were the only two drugs that had significantly lower attack rates than IFN. GLAT and Mitox were not superior to IFN. IFN with its known lacking efficacy28 34 35 was used as reference as the percentage of untreated patients was too small. The design of our study was not suitable for a direct comparison between AZA and RTX. The appearance of potential similar efficacy should therefore be interpreted with caution and currently cannot be translated into treatment recommendations.
Two previous studies had indeed suggested superiority of RTX over AZA.22 23 While our study included mainly Caucasian patients, these two other retrospective cohorts included African or predominantly Asian patients.22 23 Genetic differences have been previously suggested to contribute to the RTX therapy response in NMOSD.17 Moreover, previous studies had excluded patients with a history of previous immunosuppressive treatment. By contrast, such patients were included in our analyses, which is in line with the fact that most patients with NMOSD are treated with more than one immunosuppressant over the course of disease.5 23 In our cohort, RTX was used in >60% as second-line therapy or even later. Treatment was changed from AZA to RTX in eight patients. This could have generated a bias in favour of a low ARR in the AZA subgroup, as patients who responded well to the treatment might have remained on therapy more often as reported in another cohort;31 in addition, patients with a high ARR under AZA treatment might have been switched to RTX. Moreover, we cannot completely exclude that patients with more aggressive disease were more commonly treated with RTX than AZA based on the presumption of treatment superiority.
Our study identified predictors for therapy response independent from the chosen compound. First, the presence of AQP4-ab was identified as risk factor for attacks under therapy. Post-hoc analyses suggest that this aspect might be more important in RTX-treated than in AZA-treated patients. This is in line with a previous study that did not detect an association between attack risk and AQP-4-ab in AZA-treated and MMF-treated patients, but described a decrease of attack risk with age.36 However, the sample sizes are too small to draw final conclusions from our explorative analysis. Second, we found that a previous attack under the same therapy was associated with a 1.5-fold increased risk for further attacks. Therefore, any attack under a sufficiently dosed therapy should stipulate a discussion about alternative treatment regimes, even if the current knowledge about escalation regimes forbids a strict ‘attack equals treatment change’ algorithm. As treatment response in our cohort was completely independent from the line of therapy, suggestions on particular escalation regimes cannot be inferred. Recently, Kim et al reported in a retrospective study of 116 patients with NMOSD that non-responders to first-line therapy with AZA or MMF had less relapses on subsequent therapy with RTX.36 However, still larger and longer observations are needed to provide data on meaningful sequences of therapies. The higher risk of attacks in AQP4-ab-positive patients is in line with previous observations in untreated patients5 37 and indicates that these patients are facing higher inflammatory and more aggressive disease courses than AQP4-ab-negative patients with an NMOSD phenotype. Third, we found a decreased attack risk with increasing age. This suggests that the shorter time to disability milestones in elderly reported in the literature38 is rather driven by worse attack outcome than by higher attack rates. In accordance with this hypothesis, we found a lower remission rate with increasing age in the NEMOS cohort in our previous study.8
Using a retrospective data set, our study has several limitations. As patients with NMOSD generally require immunotherapy, we could not compare treated and untreated patients; therefore, we used the probably worst performing compound (IFN) as a reference. Second, pretreatment data could not be reliably assessed, which impeded comparison between attack risks before and during first-line treatment of NMOSD. Moreover, we cannot exclude that assignment to drugs regularly used for MS therapy might have been influenced by milder diseases courses. Third, as detailed drug dosing information was not available in all patients, treatment effects could have been underestimated or overestimated. Due to the restricted sample size, we were not able to provide differentiated efficacy estimates for all treatments or subgroup of patients. Finally, as the severity of relapses was not evaluated, differences in efficacy among the drugs could only be assessed with regard to relapse frequency. In future prospective studies, severity of relapses should be recorded.
Although we tried to compensate for other (in this regard confounding) factors when analysing HRs for each therapeutic compound, only randomised, blinded clinical trials can provide highest level evidence for therapy guidelines. However, this is difficult with NMOSD being a rare disease, and thus retrospective analyses of registry data are the currently best available option. Moreover, given the paucity of patients eligible for such prospective interventional clinical trials, it is of paramount importance to sharpen hypotheses to test in such trials using all retrospective data available. National and international collaborative initiatives are needed to engage towards prospective data collection as currently realised for the NEMOS cohort.
Acknowledgments
JPS and IK had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank all patients for participating in the study. The NEMOS cohort/NationNMO is supported by the German Ministry for Education and Research (BMBF) as part of the ‘German Competence Network Multiple Sclerosis’ (KKNMS). (FKZ 01GI1602C to JS, FKZ 01GI1602A to BW, FKZ 01GI1602B to OA)
References
Footnotes
Contributors JPS, MK, CT, and IK designed the study, collected, analysed and interpreted the data and drafted and revised the manuscript. TF did statistical analysis and revised the manuscript. AG, NB, KF, KH, FlP, KR, JH, TK OA, HPH, MR, CG, CK, AB, BH, KA, KLY, SS, MSt, FL, HT, CM, LZ, UZi, RAL, MSch, MM, FTB, UHO, ON, UZe, JHF, BW, FrP and SJ collected and analysed the data and revised the manuscript critically for intellectual content. All authors approved the final version of the manuscript.
Competing interests AB has received honoraria for consultancy or lectures and travel reimbursement from Bayer HealthCare, Biogen, Merck Serono, Mylan, Roche, Novartis and Teva, and grant support from Bayer HealthCare and Chugai. AG has received travel reimbursement from Sanofi Genzyme. BH reports grants from Chugai, grants, personal fees and non-financial support from Roche, personal fees and non-financial support from Biogen, personal fees and non-financial support from Novartis, personal fees and non-financial support from Merck, and personal fees and non-financial support from Bayer. BW has received grants from the German Ministry of Education and Research, Dietmar Hopp Foundation, Biogen, Biotest, Merck, Novartis Pharmaceuticals and Teva Pharma, personal fees from Biogen, Merck, Novartis Pharmaceuticals, Teva Pharma, Bayer HealthCare and Genzyme. CG received honoraria for lectures, travel reimbursement and grant support from Merck Serono, Teva, Novartis and CSL Behring. CT has received honoraria for consultation and expert testimony from Bayer Vital GmbH, Biogen Idec/Biogen GmbH, Genzyme GmbH and Novartis Pharmaceuticals/Pharma GmbH. FL reports travel expenses from Teva Pharma. FlP reports grants from BIH‐Charité Clinical Scientist Program funded by the Charité–Universitätsmedizin Berlin and the Berlin Institute of Health and non-financial support from ECTRIMS-Travel grant 2014. FrP reports grants and personal compensations from Alexion, Bayer, Biogen, Shire, Novartis, Medimmune, Merck and Genzyme. FTB reports grants and others from Bayer, personal fees and others from Biogen Idec, grants and personal fees from CSL Behring, grants from Fresenius, personal fees and others from Genzyme Sanofi, others from Merck Serono, grants, personal fees and others from Novartis, grants, personal fees and others from Teva, grants and others from Actelion, and grants from the German Ministry of Education and Research. HPH received, with approval of the Rector of Heinrich-Heine-University and the CEO of University of Düsseldorf Hospital, honoraria for consulting, serving on steering committees and speaking from Biogen, Geneuro, Genzyme, Medimmune, Merck, Novartis, Opexa, Receptos/Celgene, Roche, Sanofi and Teva. IK has received honoraria for consultancy or lectures and travel reimbursement from Bayer HealthCare, Biogen Idec, Chugai, Novartis, Shire and Roche, and grant support from Biogen Idec, Novartis, Chugai and Diamed. JHF received grant support and honoraria from Novartis, Bayer Vital, Merck, Biogen, Sanofi-Genzyme and Roche. JH reports personal fees and non-financial support from Sanofi Genzyme, Bayer HealthCare, Merck and Novartis Pharma. JPS received honoraria for consultancy or lectures, travel reimbursement and grant support from Biogen, Merck Serono, Novartis, Genzyme and Medimmune. KH reports grants and personal fees from Bayer HealthCare, grants and personal fees from Biogen, grants and personal fees from Teva, grants and personal fees from Merck Serono, grants and personal fees from Novartis, grants and personal fees from Almirall. KLY has nothing to disclose. KR has received research support from the German Ministry of Education and Research (BMBF/KKNMS, Competence Network Multiple Sclerosis) and Novartis, as well as speaking fees and travel grants from Guthy Jackson Charitable Foundation, Bayer HealthCare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals, Roche and Novartis. LZ has nothing to disclose. MK received grant support, travelling expenses and scientific advisory board honoraria from Novartis, Novartis Foundation, Genzyme, Bayer, Roche and Biogen. MM has received grants from Biogen, Novartis; personal fees from Bayer Vital, Biogen, Genzyme, Merck Serono, Novartis, Sanofi-Aventis and Teva; and non-financial support from Biogen. MR received speaker honoraria from Novartis and Bayer Vital GmbH, and travel reimbursement from Bayer Schering and Biogen Idec. MSch has nothing to disclose. MSt reports grants and personal fees from Bayer HealthCare, personal fees from Baxter/Baxalta, grants and personal fees from Biogen, personal fees from CSL Behring, grants and personal fees from Genzyme, personal fees from Grifols, personal fees from Merck, personal fees from Roche, grants and personal fees from Novartis, personal fees from Sanofi, and grants and personal fees from Teva. NB has received grants from Alexion Pharmaceuticals, Inc. ON has nothing to disclose. RAL reports grants and personal fees from Biogen, personal fees from Bayer, grants and personal fees from Novartis, grants and personal fees from Merck, personal fees from TEVA, personal fees from Roche, personal fees from Genzyme. SJ has received a research grant from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). MR received speaker honoraria from Novartis and Bayer Vital GmbH, and travel reimbursement from Bayer Schering and Biogen Idec. TF received honoraria for consultancies (including data monitoring committees and advisoryboards) from Novartis, Biogen, Bayer, AstraZeneca, Janssen, SGS and Pharmalog. TK reports personal fees from Biogen, grants from Novartis, personal fees from Genzyme and from Merck-Serono. UHO reports grants from Genzyme, grants from Zambon, and others from Merck Serono, Bayer, Biogen, Teva and Novartis. UZe has patents, whether planned, pending or issued, broadly relevant to the work. UZi reports personal fees from Biogen Idec GmbH, grants from Biogen Idec GmbH, personal fees from Bayer Vital GmbH, personal fees from Bristol Myers Squibb GmbH, personal fees from CorTec GmbH, personal fees from Medtronic GmbH, grants from Servier, grants from Janssen Pharmaceuticals NV and personal fees from Takeda.
Ethics approval The local institutional review boards of the participating centres approved the study (first approval from the institutional review board at Charité Universitätsmedizin EA3/004/08).
Provenance and peer review Not commissioned; externally peer reviewed.
Collaborators P Albrecht, University of Düsseldorf; O Aktas, University of Düsseldorf; K Angstwurm, University of Regensburg; I Ayzenberg, Ruhr-University Bochum; A Berthele, Technical University Munich; F Bischof, University of Tübingen; N Borisow, Charité University Medicine Berlin; T Böttcher, Bonhoeffer Klinikum Neubrandenburg; J Brettschneider, University of Ulm; M Buttmann, University of Würzburg; B Ettrich, University of Leipzig; J Faiss, Asklepios Klinik Teupitz; A Gass, University Hospital Mannheim; C Geis, University of Jena; K Guthke, Klinikum Görlitz; J Havla, Ludwig-Maximilians University Munich; H-P Hartung, University of Düsseldorf; K Hellwig, Ruhr-University Bochum; B Hemmer, Technical University Munich; F Hoffmann, Krankenhaus Martha-Maria Halle; U Hofstadt-van Oy, Klinikum Westfalen Dortmund; M Hümmert, Hannover Medical School; S Jarius, University of Heidelberg; M Kaste, Nordwest-Krankenhaus Sanderbusch; P Kermer, Nordwest-Krankenhaus Sanderbusch; P Kern, Asklepios Klinik Teupitz; C Kleinschnitz, University of Essen; I Kleiter, Ruhr-University Bochum; W Köhler, Fachkrankenhaus Hubertusburg; E Kolesilova, Asklepios Klinik Teupitz; M Krumbholz, Ludwig Maximilians University Munich; T Kümpfel, Ludwig Maximilians University Munich; S Langel, Landeskrankenhaus Rheinhessen; F Lauda, University of Ulm; M Liebetrau, Evangelische Bathildiskrankenhaus Bad Pyrmont GmbH; R Linker, University of Erlangen; W Marouf, Heliosklinik Stralsund; M Marziniak, Isar-Amper Klinik Ost Munich; S Meister, Universityof Rostock, Department of Neurology; A Melms, University of Erlangen; I Metz, University of Göttingen; C Mayer, University of Frankfurt; C Münch, Charité University Medicine Berlin; O Neuhaus, SRH Krankenhaus Sigmaringen; S Niehaus, Klinikum Dortmund; F Pache, Charité University Medicine Berlin; F Paul, Charité University Medicine Berlin; H Pellkofer, University of Göttingen; A Riedlinger, Asklepios Klinik Teupitz; M Ringelstein, University of Düsseldorf; L Röpke, University of Jena; SP Rommer, University of Vienna (Austria); K Ruprecht, Charité University Medicine Berlin; C Ruschil, University of Tübingen; S Schippling, University of Zürich (Switzerland); S Schuster, University ofHamburg; M Schwab, University of Jena; M Stangel, Hannover Medical School; J Stellmann, University of Hamburg; M Stoppe, University of Leipzig; F Then Bergh, University of Leipzig; C Trebst, Hannover Medical School; J Tünnerhoff, University of Tübingen; H Tumani, University of Ulm; C Veauthier, Charité University Medicine Berlin; A Walter, Klinikum Herford; KP Wandinger, Institute of Clinical Chemistry, Neuroimmunology Unit, and Department of Neurology, University Medical Center Schleswig-Holstein Campus Lübeck; MS Weber, University of Göttingen; R Weissert, University of Regensburg; B Wildemann, University of Heidelberg; C Wilke, Nervenzentrum Potsdam; A Winkelmann, University of Rostock, Department of Neurology; K Young, University of Hamburg; L Zeltner, University of Tübingen; C Zentner, Krankenhaus Martha-Maria Halle; U Zettl, University of Rostock, Department of Neurology, Neuroimmunological Section; U Ziemann, University of Tübingen.