Summary
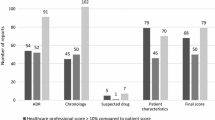
Three clinical pharmacologists independently evaluated 500 untoward clinical events reported by physicians as adverse drug reactions (ADRs). They often disagreed with the reporting physicians and with each other. They judged 14.4–28.2% of the events to be definite, 26.4–38.0% probable, 21.4–31.0% possible and 15.8–28.2% unlikely ADRs. In their opinion 19.1–32.4% of the drugs blamed were definitely, 29.8–34.4% probably, 24.9–36.7% possibly and 8.4–14.5% not responsible for the adverse reactions. Evaluators disagreed among themselves about the drug most likely to have been responsible in 36.4% of the events, about ADRs causing hospital admission in 56.8%, about severe ADR morbidity in 55.8%, about ADR prolongation of hospitalization in 67.3% and about ADR contribution to death in 71.0%. The divergence of judgements suggests that suspected ADRs are usually ambiguous clinical events, and that incidence, severity, medical consequences and cost of ADRs can only be estimated.
Similar content being viewed by others
References
McQueen, E. G.: Pharmacologic basis of adverse drug reactions. In: Drug treatment (ed. G.S. Avery), pp. 161–192. Sidney: Adis Press 1976
Feinstein, A. R.: Clinical biostatistics. XXVII. The biostatistical problems of pharmaceutical surveillance. Clin. Pharmacol. Ther.16, 110–123 (1974)
Karch, F. E., Lasagna, L.: Adverse drug reactions. A critical review. J. Amer. med. Ass.234, 1236–1241 (1975)
Koch-Weser, J.: Detection and evaluation of adverse drug reactions. In: Clinical pharmacy and pharmacology (ed. W. A. Gouveia, G. Tognoni). Amsterdam: Excerpta Medica (in press)
Koch-Weser, J., Sidel, V. W., Sweet, R. H., Kanarek, P., Eaton, A.: Factors determining physician reporting of adverse drug reactions. Comparison of 2000 spontaneous reports with surveillance studies at the Massachusetts General Hospital. New Engl. J. Med.280, 20–26 (1969)
Koch-Weser, J.: Definition and classification of adverse drug reactions. Drug Informat. Bull.2, 72–78 (1968)
Miller, R. R., Greenblatt, D. J. (ed): Drug effects in hospitalized patients. Experiences of the Boston Collaborative Drug Surveillance Program 1966–1975. New York: John Wiley & Sons 1976
Koch-Weser, J.: Fatal reactions to drug therapy. New Engl. J. Med.291, 302–303 (1974)
Irey, N. S.: Adverse drug reactions and death. A review of 827 cases. J. Amer. med. Ass.236, 575–578 (1976)
Karch, F. E., Smith, C. L., Kerzner, B., Mazullo, J. M., Weintraub, M., Lasagna, L.: Adverse drug reactions — a matter of opinion. Clin. Pharmacol. Ther.19, 482–492 (1976).
Karch, F. E., Lasagna, L.: Adverse drug reactions in the United States. An analysis of the scope of the problem and recommendations for future approaches. Washington: Medicine in the Public Interest 1974
Green, D. M.: Pre-existing conditions, placebo reactions, and “side-effects”. Ann. intern. Med.60, 255–265 (1964)
Reidenberg, M. M., Lowenthal, D. T.: Adverse nondrug reactions. New Engl. J. Med.279, 678–679 (1968)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koch-Weser, J., Sellers, E.M. & Zacest, R. The ambiguity of adverse drug reactions. Eur J Clin Pharmacol 11, 75–78 (1977). https://doi.org/10.1007/BF00562895
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00562895