Summary
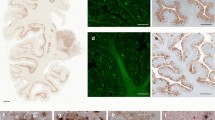
We examined paraffin-embedded brain sections from three patients with Creutzfeldt-Jakob disease (CJD) and four patients with Gerstmann-Sträussler syndrome (GSS) who also had β protein deposits in the brains. Immunostaining using anti-prion protein (PrP) and anti-β protein coupled with formic acid pretreatment, revealed PrP deposits and β protein deposits, respectively. In all four GSS patients examined, sequential double immunostaining and single immunostaining in serial sections or simultaneous double immunofluorescence revealed the colocalization of PrP and β protein in the same amyloid plaques. The plaques labeled with both antibodies were designated as β-PrP plaques. Small kuru plaques of less than 15 μm in diameter were rarely found to coexist with β deposits. The percentages of β-PrP plaques in larger kuru plaques were not constant among the four GSS patients. The colocalization patterns of both deposits were observed as being roughly of two types as follows: (1) diffuse β protein deposits located around the PrP core; and (2) a β protein core and PrP core simultaneously existing in one amyloid plaque. Under an electron microscope, we were able to confirm the presence of both β protein and PrP in a single plaque in four GSS patients older than 60 years old. In contrast, no colocalization of either deposits was seen in the amyloid plaque core fractions of a young GSS patient who had no β protein deposits, even at the electron microscopic level. Therefore, the colocalization of both proteins in a single plaque is believed to be age-related and incidental in GSS patients but suggests a similar morphogenesis of both amyloid deposits.
Similar content being viewed by others
References
Altman LG, Schneider BG, Papermaster DS (1984) Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem 32:1217–1223
Brown P, Jannotta F, Gibbs CJ, Baron H, Guiroy DC, Gajdusek DC (1990) Coexistence of Creutzfeldt-Jakob disease and Alzheimer's disease in the same patient. Neurology 40:226–228
DeArmond SJ, McKinley MP, Barry RA, Braunfeld MB, McColloch JR, Prusiner SB (1985) Identification of prion amyloid filaments in scrapie-infected brain. Cell 41:221–235
Doerr-schott J, Kitamoto T, Tateishi J, Boellaard JW, Heldt N, Lichte C (1990) Technical communication: immunogold light and electron microscopic detection of amyloid plaques in transmissible spongiform encephalopathies. Neuropathol Appl Neurobiol 16:85–89
Doh-ura K, Tateishi J, Sasaki H, Kitamoto T, Sakaki Y (1989) Pro-Leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun 163:974–979
Doh-ura K, Tateishi J, Kitamoto T, Sasaki Y (1990) Creutzfeldt-Jakob disease patients with congophilic kuru plaques have the missense variant prion protein common to Gerstmann-Sträussler syndrome. Ann Neurol 27:121–126
Gaches J, Supino-Viterbo V (1977) Association de maladies d'Alzheimer et de Creutzfeldt-Jakob. Acta Neurol Belg 77:202–212
Glaccone G, Tagliavini F, Verga L, Frangione B, Farlow MR, Ghetti B, Bugiani O (1991) Coexistence of prion protein and β-protein in an aged patient with Gerstmann-Sträussler-Scheinker disease. Neurology [Suppl 1] 41:155
Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Guesdon JL, Ternynck T, Avrameas S (1979) The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem 27:1131–1139
Hsu SM, Soban E (1982) Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem 30:1079–1082
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup P, Beyreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325:733–736
Kitamoto T, Tateishi J (1988) Immunohistochemical confirmation of Creutzfeldt-Jakob disease with a long clinical course with amyloid plaque core antibodies. Am J Pathol 131:435–443
Kitamoto T, Ogomori K, Tateishi J, Prusiner SB (1987) Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest 57:230–236
Kitamoto T, Tateishi J, Sawa H, Doh-ura K (1989) Positive transmission of Creutzfeldt-Jakob disease verified by murine kuru plaques. Lab Invest 60:507–512
Kitamoto T, Yamaguchi K, Doh-ura K, Tateishi J (1991) A prion protein missense variant is integrated in kuru plaque cores in patients with Gerstmann-Sträussler syndrome. Neurology 41:306–310
Liberski PP, Papeerz W, Alwasiak J (1987) Creutzfeldt-Jakob disease with plaques and paired helical filaments. Acta Neurol Scand 76:428–432
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Miyazono M, Iwaki T, Kitamoto T, Kaneko Y, Doh-ura K, Tateishi J (1991) A comparative immunohistochemical study of kuru and senile plaques with a special reference to glial reactions at various stages of amyloid plaque formation. Am J Pathol 139:589–598
Oesch B, Westaway D, Walchli M, McKinley MP, Kent SBH, Aebersold R, Barry RA, Tempet P, Teplow DB, Hood LE, Prusiner SB, Weismann C (1985) A cellular gene encodes scrapie PrP 27–30 protein. Cell 40:735–746
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Roberts GW, Lofthouse R, Allsop D, Landon M, Kidd M, Prusiner SB, Crow TJ (1988) CNS amyloid proteins in neurodegenerative diseases. Neurology 38:1534–1540
Tateishi J, Kitamoto T, Hashiguchi H, Shii H (1988) Gerstmann-Sträussler-Scheinker disease. Immunohistological and experimental studies. Ann Neurol 24:35–40
Tateishi J, Ohta M, Koga M, Sato Y, Kuroiwa Y (1979) Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol 5:581–584
Wiley CA, Burrola PG, Buchmeier MJ, Wooddell MK, Barry RA, Prusiner SB, Lampert PW (1987) Immuno-gold localization of prion filaments in scrapie-infected hamster brains. Lab Invest 57:646–656
Author information
Authors and Affiliations
Additional information
Supported in part by a Grant-in-Aid for Scientific Research (02454245, 03454171) from the Ministry of Education, Science and Culture of Japan
Rights and permissions
About this article
Cite this article
Miyazono, M., Kitamoto, T., Iwaki, T. et al. Colocalization of prion protein and β protein in the same amyloid plaques in patients with Gerstmann-Sträussler Syndrome. Acta Neuropathol 83, 333–339 (1992). https://doi.org/10.1007/BF00713522
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00713522