Summary
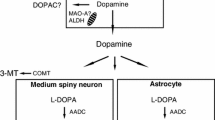
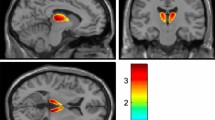
The effect of peripheral catechol-O-methyltransferase (COMT) inhibition with entacapone on striatal uptake of 6-[18F]fluoro-L-dopa (FDOPA) was studied with PET both without and with entacapone in fifteen advanced parkinsonian patients and six healthy controls. Entacapone significantly enhanced the fraction of unmetabolized FDOPA in plasma from 16% to about 50% at 80 minutes after FDOPA injection in all subjects. The striatal to occipital ratios and the striatal FDOPA uptake, expressed as a modified decarboxylation coefficient (k3R0), was significantly increased in healthy controls, whereas in parkinsonian patients the increase was significant only in the caudate. On the other hand, the influx constant (Ki) decreased significantly in the caudate and putamen in parkinsonian patients; in healthy controls the Ki remained virtually unchanged.
Effective peripheral COMT inhibition markedly increased the fraction of FDOPA in plasma and thus its availability in the brain for decarboxylation both in patients and control subjects. However, the change in striatal FDOPA uptake was modest in the advanced parkinsonian patients as compared to that in control subjects, because of the advanced disease, decreased storage capacity, or both.
Similar content being viewed by others
References
Ahtila S, Kaakkola S, Gordin A, Korpela K, Heinävaara S, Karlsson M, Wikberg T, Tuomainen P, Männistö PT (1995) Effect of entacapone, a COMT inhibitor, on the pharmacokinetics and metabolism of levodopa after administration of controlled-release levodopa-carbidopa in volunteers. Clin Neuropharmacol 18: 46–57
Aquilonius S-M, Eckernäs S-Å (1980) A colour atlas of the human brain. Esselte studium, Stockholm, Sweden
Bergman J, Haaparanta M, Solin O (1993) 6-[18F]Fluoro-L-Dopa: synthesis and metabolite studies. In: Heselius S-J, Lill J-O, Martin J (eds) The Åbo Akademi Accelerator Laboratory Triennal Report 1990–92. Turku, Finland 1, pp 80–85
Bergman J, Haaparanta M, Lehikoinen P, Solin O (1994) Electrophilic synthesis of 6-[18F]fluoro-L-dopa, starting from aqueous [18F]-fluoride. J Label Compounds Radiopharm 35: 476–477
Boyes BE, Cumming P, Martin WRW, McGeer EG (1986) Determination of plasma [18F]-6-fluorodopa during positron emission tomography: elimination and metabolism in carbidopa treated subjects. Life Sci 39: 2243–2252
Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, Marsden CD, Frackowiak RSJ (1990) The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson's disease, studied with PET. Brain 113: 1539–1552
Chirakal R, Firnau G, Garnett ES (1986) High yield synthesis of 6-[18F]fluoro-L-dopa. J Nucl Med 27: 417–421
Chiueh CC, Firnau G, Burns RS, Nahmias C, Chirakal R, Kopin IJ, Garnett ES (1986) Determination and visualization of damage to striatal dopaminergic terminals in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism by [18F]-labeled 6-fluoro-L-DOPA and positron emission tomography. Adv Neurol 45: 167–169
Creveling CR, Kirk KL (1985) The effect of ring-fluorination on the rate of O-methylation of dihydroxyphenylalanine (DOPA) by catechol-O-methyltransferase: significance in the development of18F-PETT scanning agents. Biochem Biophys Res Commun 130: 1123–1131
Doudet DJ, McLellan CA, Carson R, Adams HR, Miyake H, Aigner TG, Finn RT, Cohen RM (1991) Distribution and kinetics of 3-O-methyl-6-[18F]fluoro-L-DOPA in the rhesus monkey brain. J Cereb Blood Flow Metab 11: 726–734
Firnau G, Sood S, Chirakal R, Nahmias C, Garnett ES (1987) Cerebral metabolism of 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine in the primate. J Neurochem 48: 1077–1082
Garnett ES, Firnau G, Chan PKH, Sood S, Belbeck LW (1978) [18F]Fluoro-dopa, an analogue of dopa, and its use in direct external measurements of storage, degradation, and turnover of intracerebral dopamine. Proc Natl Acad Sci USA 75: 464–467
Garnett ES, Firnau G, Nahmias C (1983) Dopamine visualized in the basal ganglia of living man. Nature 305: 137–138
Gjedde A (1982) Calculation of cerebral glucose phosphorylation from brain uptake of glucose analogs in vivo: a re-examination. Brain Res Rev 4: 237–274
Guttman M, Léger G, Cedarbaum JM, Reches A, Woodward W, Evans A, Diksic M, Gjedde A (1992) 3-O-methyldopa administration does not alter fluorodopa transport into the brain. Ann Neurol 31: 638–643
Guttman M, Léger G, Reches A, Evans A, Kuwabara H, Cedarbaum JM, Gjedde A (1993) Administration of the new COMT inhibitor OR-611 increases striatal uptake of fluorodopa. Mov Disord 8: 298–304
Günther I, Psylla M, Antonini A, Haeberli M, Reddy G, Beer HF, Leenders KL (1993) Striatal [18F]Fluoro-L-Dopa (FD) uptake and arterial plasma tracer metabolite alterations as a result of catechol-O-methyl transferase (COMT) and L-aromatic-amino-acid-decarboxylase (AAAD) inhibition. Neurology 43 [Suppl 2]: A196
Hartvig P, Ågren H, Reibring L, Tedroff J, Bjurling P, Kihlberg T, Långström B (1991) Brain kinetics of L-[β-11C)-L-DOPA in humans studied by positron emission tomography. J Neural Transm [Gen Sect] 86: 25–41
Hartvig P, Lindner KJ, Tedroff J, Bjurling P, Hörnfelt K, Långström B (1992) Regional brain kinetics of 6-fluoro-(β-11C)-L-DOPA and (β-11C)-L-DOPA following COMT inhibition. A study in vivo using positron emission tomography. J Neural Trasm [Gen Sect] 87: 15–22
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 17: 427–442
Hoffman JM, Melega WP, Hawk TC, Grafton SC, Luxen A, Mahoney DK, Barrio JR, Huang S-C, Mazziotta JC, Phelps ME (1992) The effects of carbidopa administration on 6-[18F]fluoro-L-DOPA kinetics in positron emission tomography. J Nucl Med 33: 1472–1477
Hoshi H, Kuwabara H, Léger G, Cumming P, Guttman M, Gjedde A (1993) 6-[18F]fluoro-L-DOPA metabolism in living human brain: a comparison of six analytical methods. J Cereb Blood Flow Metab 13: 57–69
Kaakkola S, Teräväinen H, Ahtila S, Rita H, Gordin A (1994) Effect of entacapone, a COMT inhibitor, on clinical disability and levodopa metabolism in parkinsonian patients. Neurology 44: 77–80
Kuruma I, Bartholini G, Tissot R, Pletscher A (1971) The metabolism of L-3-O-methyldopa, a precursor of dopa in man. Clin Pharmacol Ther 12: 678–682
Kuwabara H, Cumming P, Reith J, Leger G, Diksic M, Evans AC, Gjedde A (1993) Human striatal L-DOPA decarboxylase activity estimated in vivo using 6-[18F]fluoro-DOPA and positron emission tomography: error analysis and application to normal subjects. J Cereb Blood Flow Metab 13: 43–56
Laihinen A, Rinne JO, Rinne UK, Haaparanta M, Ruotsalainen U, Bergman J, Solin O (1992) [18F]-6-fluorodopa PET scanning in Parkinson's disease after selective COMT inhibition with nitecapone (OR-462). Neurology 42: 199–203
Leeders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, Nahmias C, Jones T, Marsden CD (1986) Brain dopamine metabolism in patients with Parkinson's disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry 49: 853–860
Martin WRW, Stoessl AJ, Adam MJ, Ammann W, Bergstrom M, Harrop R, Laihinen A, Rogers JG, Ruth TJ, Sayre CI, Pate BD, Calne DB (1986) Positron emission tomography in Parkinson's disease: glucose and DOPA metabolism. Adv Neurol 45: 95–98
Melega WP, Luxen A, Perlmutter MM, Nissenson CHK, Phelps ME, Barrio JR (1990) Comparative in vivo metabolism of 6-[18F]fluoro-L-DOPA and [3H]L-DOPA in rats. Biochem Pharmacol 39: 1853–1860
Melega WP, Grafton ST, Huang S-C, Satyamurthy N, Phelps ME, Barrio JR (1991) L-6-[18F]fluoro-DOPA metabolism in monkeys and humans: biochemical parameters for the formulation of tracer kinetic models with positron emission tomography. J Cereb Blood Flow Metab 11: 890–897
Merello M, Lees AJ, Webster R, Bovingdon M, Gordin A (1994) Effect of entacapone, a peripherally acting catechol-O-methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 57: 186–189
Namavari M, Bishop A, Satyamurthy N, Bida G, Barrio JR (1992) Regioselective radiofluoro destannylation with [18F]F2 and [18F]CH3COOF: a high yield synthesis of 6-[18F]fluoro-L-dopa. Int J Appl Radiat Isot 43: 989–996
Nissinen E, Lindén I-B, Schultz E, Pohto P (1992) Biochemical and pharmacological properties of a peripherally acting catechol-O-methyltransferase inhibitor Entacapone. Naunyn Schmiedebergs Arch Pharmacol 346: 262–266
Nutt JG, Fellman JH (1984) Pharmacokinetics of levodopa. Clin Neuropharmacol 7: 35–49
Nutt JG, Woodward WR, Beckner RM, Stone CK, Berggren K, Carter JH, Gancher ST, Hammerstad JP, Gordin A (1994) Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology 44: 913–919
Patlak CS (1981) Derivation of equations for the steady-state reaction velocity of a substance based on the use of a second substance. J Cereb Blood Flow Metab 1: 129–131
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3: 1–7
Ruottinen HM, Rinne UK (1995) Effect of one month's treatment with peripherally acting COMT inhibitor, entacapone, on pharmacokinetics and motor response to levodopa in advanced parkinsonian patients. Clin Neuropharmacol (in press)
Ruottinen H, Rinne UK, Ahtila S, Gordin A (1994) A dose-finding clinical and pharmacokinetic study of entacapone as an adjuvant to levodopa treatment in Parkinson's disease. Neurology 44 [Suppl 2]: A258
Sawle GV, Leenders KL, Brooks DJ, Harwood G, Lees AJ, Frackowiak RSJ, Marsden CD (1991) Dopa-responsive dystonia: [18F]dopa positron emission tomography. Ann Neurol 30: 24–30
Sawle GV, Burn DJ, Morrish PK, Lammertsma AA, Snow BJ, Luthra S, Osman S, Brooks DJ (1994) The effect of entacapone (OR-611) on brain [18F]-6-fluorodopa metabolism: implications for levodopa therapy of Parkinson's disease. Neurology 44: 1292–1297
Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, Kimura H (1993) Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol 34: 324–330
Spinks TJ, Jones T, Gilardi MC, Heather JD (1988) Physical performance of the latest generation of commercial positron scanner. IEEE Trans Nucl Med 35: 721–725
Tedroff J, Aquilonius S-M, Laihinen A, Rinne U, Hartvig P, Andersson J, Lundqvist H, Haaparanta M, Solin O, Antoni G, Gee AD, Ulin J, Långström B (1990) Striatal kinetics of [11C]-(+)-nomifensine and 6-[18F]fluoro-L-dopa in Parkinson's disease measured with positron emission tomography. Acta Neurol Scand 81: 24–30
Wade LA, Katzman R (1975) 3-O-methyldopa uptake and inhibition of L-dopa at the blood-brain barrier. Life Sci 17: 131–136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ruottinen, H.M., Rinne, J.O., Ruotsalainen, U.H. et al. Striatal [18F]fluorodopa utilization after COMT inhibition with entacapone studied with PET in advanced Parkinson's disease. J Neural Transm Gen Sect 10, 91–106 (1995). https://doi.org/10.1007/BF02251225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02251225