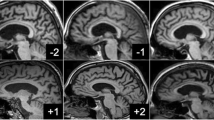
Summary
We studied the usefulness of measuring volumes of the hippocampus, amygdala and frontal lobes with coronal magnetic resonance imaging (MRI) scans in the diagnosis of early Alzheimer's disease (AD). We examined 32 patients diagnosed according to the NINCDS-ADRDA criteria of probable AD and 16 age-matched healthy cognitively normal controls. The AD patients had mild dementia with a mean score of 22.8 in the Mini-Mental Status Examination (MMSE). We used a 1.5T magnetic resonance imager and normalized the volumes for brain area. The AD patients had significantly smaller volumes of the right and the left hippocampus (−38%) (ANOVA, p<0.001) and the left frontal lobe (−16%, p<0.05) compared to controls. The reductions in volumes of the right frontal lobe (−13%), the right amygdala (−14%) or the left amygdala (−18%) were not statistically significant. In the discriminant function analysis which included the volumes of the hippocampus, amygdala, and the frontal lobes and age, the volumes of the left and right hippocampus, the left and right frontal lobe, and the right amygdala entered the model and we could correctly classify 92% of the subjects into AD and control groups (Chi-square 42.6, df 5, p<0.0001). By using the volumes of the hippocampus, the frontal lobes or the amygdala on their alone, the correct classification was achieved in 88%, 65% and 58% of the subjects, respectively. In addition, in AD patients the volumes of the left hippocampus correlated significantly with the MMSE score and with immediate and delayed verbal memory; the smaller the volume the more impaired was their performance. Our data indicate that measurements of volumes of the hippocampus might be useful in diagnosis of early AD.
Similar content being viewed by others
References
Borkowski JG, Benton AL, Spreen O (1967) Word fluency and brain damage. Neuropsychologia 5: 135–140
Bottomley PA, Cousins JP, Pendrey DL, Wagle WA, Hardy CJ, Eames FA, McCaffrey RJ, Thompson DA (1992) Alzheimer dementia: quantification of energy metabolism and mobile phosphoesters with P-31 NMR spectroscopy. Radiology 183: 695–699
Bronen RA, Cheung G (1991a) MRI of the temporal lobe: normal variations, with special reference toward epilepsy. Magn Reson Imaging 9: 501–507
Bronen RA, Cheung G (1991b) Relationship of hippocampus and amygdala to coronal MRI landmarks. Magn Reson Imaging 9: 449–457
Coffey CE, Wilkinson WE, Parashos IA, Soady SAR, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT (1992) Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology 42: 527–536
Cuénod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F (1993) Amygdala atrophy in Alzheimer's disease. Arch Neurol 50: 941–945
Dahlbeck SW, McCluney KW, Yeakley JW, Fenstermacher MJ, Bonmati C, Van Horn III G, Aldag J (1991) The interuncal distance: a new MR measurement for the hippocampal atrophy of Alzheimer's disease. AJNR 12: 931–932
Davis PC, Gray L, Albert M, Wilkinson W, Hughes J, Heyman A, Gado M, Kumar AJ, Destian S, Lee C, Duvall E, Kido D, Nelson MJ, Bello J, Weathers S, Jolesz F, Kikinis R, Brooks M (1992) The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part III. Reliability of standardized MRI evaluation of Alzheimer's disease. Neurology 42: 1676–1680
DeArmond SJ, Fusco MM, Dewey MM (1989) Structure of the human brain. A photographic atlas, 3rd ed. Oxford University Press, New York
DeCarli C, Kaye JA, Horwitz B, Rapoport SI (1990) Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology 40: 872–883
Doraiswamy PM, McDonald WM, Patterson L, Husain MM, Figiel GS, Boyko OB, Krishnan KR (1993) Interuncal distance as a measure of hippocampal atrophy: normative data on axial MR imaging. AJNR 14: 141–143
Duvernoy HM (1988) The human hippocampus: an atlas of applied anatomy. J.F. Bergmann, Munich
Erkinjuntti T, Lee DH, Gao F, Steenhuis R, Eliasziw M, Fry R, Merskey H, Hachinski V (1993) Temporal lobe atrophy on magnetic resonance imaging in the diagnosis of early Alzheimer's disease. Arch Neurol 50: 305–310
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198
Goodglass H, Kaplan E (1972) The assessment of aphasia and related disorders. Lea and Febiger, Philadelphia
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62
Haxby JV, Grady CL, Duara R, Schlageter N, Berg G, Rapoport SI (1986) Neocortical metabolic abnormalities precede nonmemory cognitive defects in early Alzheimer'stype dementia. Arch Neurol 43: 882–885
Helkala E-L, Laulumaa V, Soininen H, Riekkinen PJ (1988) Recall and recognition on memory in patients with Alzheimer's and Parkinson's diseases. Ann Neurol 24: 214–217
Huesgen CT, Burger PC, Crain BJ, Johnson GA (1993) In vitro MR microscopy of the hippocampus in Alzheimer's disease. Neurology 43: 145–152
Hyman BT, Damasio AR, VanHoesen GW, Barnes CL (1984) Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 225: 1168–1170
Jack CR, Sharbrough FW, Colleen KT, Cascino GD, Hirschorn KA, Marsh WR, Zinsmeister AR, Scheithauer B (1990) Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 175: 423–429
Jack CR Jr, Petersen RC, O'Brien PC, Tangalos EG (1992) MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 42: 183–188
Jernigan TJ, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR (1991) Cerebral structure on MRI, part I. Localization of age-related changes. Biol Psychiatry 29: 55–67
Kaplan E, Goodglass H, Weintraub S (1983) The Boston naming test. Lea and Febiger, Philadelphia
Kesslak JP, Nalcioglu O, Cotman CW (1991a) Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology 41: 51–54
Kesslak JP, Nalcioglu O, Cotman W (1991b) MRI quantification (a letter). Neurology 41: 954
Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F (1993) Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Arch Neurol 50: 949–954
Kirsch SJ, Jacobs RW, Butcher LL, Beatty J (1992) Prolongation of magnetic resonance T2 time in hippocampus of human patients marks the presence and severity of Alzheimer's disease. Neurosci Lett 134: 187–190
Klunk WE, Panchalingam K, Moossy J, McClue RJ, Pettegrew JW (1992) N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer's disease brain: a preliminary proton nuclear magnetic resonance study. Neurology 42: 1578–1585
Krishnan KRR, Husain MM, McDonald WM, Doraiswamy PM (1990) In vivo stereological assessment of caudate nuclei volume with MRI: effect of normal aging. Life Sci 47: 1325–1329
Longo R, Giorgini A, Magnaldi S, Pascazio L, Ricci C (1993) Alzheimer's disease histologically proven studied by MRI and MRS: two cases. Magn Reson Imaging 11: 1209–1215
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer's disease: report of NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34: 939–944
Millber WP, Hebben N, Kaplan E (1986) The Boston process approach to neuropsychological assessment. In: Grant I, Adams KM (eds) Neuropsychological assessment of neuropsychiatric disorders. Oxford University Press, New York, pp 65–86
Miller LA, Munoz DG, Finmore M (1993) Hippocampal sclerosis and human memory. Arch Neurol 50: 391–394
Murphy DGM, DeGarli C, Schapiro MB, Rapoport SI, Horwitz B (1992) Age-related differences in volumes of subcortical nuclei, brain matter and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol 49: 839–845
Naidich TP, Daniels DL, Haughton VM, Williams A, Pojunas K, Palacios E (1987) Hippocampal formation and related structures of the limbic lobe: anatomic-MR correlation. Radiology 162: 747–754
Nelson HE (1976) A modified card sorting test sensitive to frontal lobe defects. Cortex 12: 313–324
Pearlson GD, Gordon JH, Powers RE, Barta PE, Camargo EE, Chase GA, Noga JT, Tune LE (1992) Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry 49: 402–408
Press GA, Amaral DG, Squire LR (1989) Hippocampal abnormalities in amnesic patients revealed by high-resolution magnetic resonance imaging. Nature 341: 54–57
Reisberg B, Schenck MK, Ferris SH (1983) The brief cognitive rating scale (BCRS): findings in primary degenerative dementia (PDD). Psychopharmacol Bull 19: 734–739
Reitan RM (1958) Validity of the Trail Making test as an indicator of organic brain damage. Perc Mot Skills 8: 271–276
Rosen WG, Terry RD, Fuld PA, Katzman R, Beck A (1980) Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 17: 486–488
Russel FW (1975) A multiple scoring method for the assessment of complex memory functions. J Cons Clin Psychology 43: 800–809
Scott SA, DeKosky ST, Scheff W (1991) Volumetric atrophy in Alzheimer's disease: quantitative serial reconstruction. Neurology 41: 351–356
Seab JP, Jagust WJ, Wong STS, Roos MS, Reed BR, Budinger TF (1988) Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magn Reson Med 8: 200–208
Soininen H, Partanen K, Pitkänen A, Vainio P, Hänninen T, Hallikainen M, Koivisto K, Riekkinen PJ Sr (1994) Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology 44: 1660–1668
Squire LR, Ojeman JG, Miezin FM, Petersen SE, Videen TO, Raichle ME (1992) Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci USA 89: 1837–1841
Suddath RL, Christinson GW, Torrey EF, Casanova MF, Weinberger DR (1990) Anatomic anomalies in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 322: 789–794
Sulkava R, Haltia M, Paetau A, Wikström J, Palo J (1983) Accuracy of clinical diagnosis in primary degenerative dementia: correlation with neuropathological findings. J Neurol Neurosurg Psychiatry 46: 9–13
Tien RD, Felsberg GJ, Crain B (1992) Normal anatomy of the hippocampus and adjacent temporal lobe: high resolution fast spin echo MR images in volunteers correlated with cadaveric histologic sections. AJR 159: 1309–1313
Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G (1992) Anatomical basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 42: 1743–1750
Webster DD (1968) Clinical analysis of the disability on Parkinson's disease. Mod Treat 5: 257–262
Wechsler D (1981) WAIS-R Manual. Psychological Corporation, New York
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal aging and Alzheimer's disease. Lancet 344: 769–772
Zola-Morgan S, Squire LR, Mishkin M (1982) The neuroanatomy of amnesia: amygdalahippocampus vs temporal stem. Science 218: 1337–1339
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laakso, M.P., Soininen, H., Partanen, K. et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: Correlation with memory functions. J Neural Transm Gen Sect 9, 73–86 (1995). https://doi.org/10.1007/BF02252964
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02252964