Abstract
It has been shown that high-frequency repetitive transcranial magnetic stimulation (rTMS) to the human primary motor hand area (M1-HAND) can induce a lasting increase in corticospinal excitability. Here we recorded motor evoked potentials (MEPs) from the right first dorsal interosseus muscle to investigate how sub-threshold high-frequency rTMS to the M1-HAND modulates cortical and spinal excitability. In a first experiment, we gave 1500 stimuli of 5 Hz rTMS. At an intensity of 90% of active motor threshold, rTMS produced no effect on MEP amplitude at rest. Increasing the intensity to 90% of resting motor threshold (RMT), rTMS produced an increase in MEP amplitude. This facilitatory effect gradually built up during the course of rTMS, reaching significance after the administration of 900 stimuli. In a second experiment, MEPs were elicited during tonic contraction using weak anodal electrical or magnetic test stimuli. 1500 (but not 600) conditioning stimuli at 90% of RMT induced a facilitation of MEPs in the contracting FDI muscle. In a third experiment, 600 conditioning stimuli were given at 90% of RMT to the M1-HAND. Using two well-established conditioning-test paradigms, we found a decrease in short-latency intracortical inhibition (SICI), and a facilitation of the first peak of facilitatory I-waves interaction (SICF). There was no correlation between the relative changes in SICI and SICF. These results demonstrate that subthreshold 5 Hz rTMS can induce lasting changes in specific neuronal subpopulations in the human corticospinal motor system, depending on the intensity and duration of rTMS. Short 5 Hz rTMS (600 stimuli) at 90% of RMT can selectively shape the excitability of distinct intracortical circuits, whereas prolonged 5 Hz rTMS (≥900 stimuli) provokes an overall increase in excitability of the corticospinal output system, including spinal motoneurones.
Similar content being viewed by others
Introduction
In recent years, repetitive transcranial magnetic stimulation (rTMS) of the human neocortex has been increasingly used to produce effects on cortical circuits that outlast the duration of rTMS itself (for a review, see Siebner and Rothwell 2003). The conditioning effects of rTMS have been mainly studied in the primary motor hand area (M1-HAND) because overall changes in corticomotor excitability can be readily assessed with TMS by measuring the size of the motor evoked response (MEP) in the contralateral upper limb (Chen et al. 1997; Pascual-Leone et al. 1994). However, a change in MEP size might be caused by changes in excitability at the cortical or spinal level, or both. In contrast to single-pulse TMS, paired-pulse TMS is better suited to assessing specific changes in excitability in the stimulated M1-HAND. Indeed, several conditioning-test paradigms have been established that allow us to probe the aftereffects of rTMS on specific intracortical circuits in the stimulated M1-HAND (Kujirai et al. 1993; Tokimura et al. 1996; Rothwell 1999; Ziemann et al. 1998a; Ziemann 1999).
There is converging evidence to suggest that the frequency of rTMS plays a critical role in defining the direction of changes in corticospinal excitability. At intensities above resting motor threshold, high-frequency rTMS (referring to rTMS given at frequencies of about 5 Hz and above) tends to produce an increase in corticospinal excitability (Pascual-Leone et al. 1994; Wu et al. 2000). Conversely, suprathreshold low-frequency rTMS (referring to rTMS given at frequencies of about 1 Hz) tends to reduce corticospinal excitability (Chen et al. 1997; Muellbacher et al. 2000). Because rTMS at suprathreshold stimulus intensities provokes repetitive muscle twitches in the contralateral limb, some of the aftereffects may have been caused by repetitive sensory feedback activation during rTMS. However, similar frequency-dependent effects have also been found at subthreshold intensities, suggesting that conditioning effects were directly induced by rTMS of the M1-HAND (Maeda et al. 2000a; Gangitano et al. 2002).
Using short trains of rTMS, Modugno et al. (2001) were the first to show that, in addition to the frequency, other variables of rTMS, such as the intensity and the number of stimuli, shape the aftereffects of rTMS. A number of recent studies have investigated in more detail the conditioning effects of prolonged (≥900 stimuli) low-frequency (1 Hz) rTMS to the M1-HAND on corticospinal (Maeda et al. 2000a, 2000b; Müllbacher et al. 2000; Touge et al. 2001; Fitzgerald et al. 2002), spinal (Touge et al. 2001; Valero-Cabre et al. 2001) and intracortical excitability (Fitzgerald et al. 2002; Romero et al. 2002). These studies demonstrated that the number of stimuli (Maeda et al. 2000a; Touge et al. 2001), the intensity of stimulation (Fitzgerald et al. 2002), and the pulse configuration (Sommer et al. 2002) have an impact on the changes in excitability that can be observed after rTMS.
To date, only a relatively small number of rTMS studies have addressed the conditioning effects of subthreshold high-frequency rTMS on corticospinal excitability (Maeda et al. 2000a, 2000b; Peinemann et al. 2000; Di Lazzaro et al. 2002). These studies suggest that high-frequency rTMS can induce two distinct changes in corticomotor excitability. Subthreshold high-frequency rTMS can selectively decrease intracortical paired-pulse inhibition in the M1-HAND (Pascual-Leone et al. 1998; Peinemann et al. 2000; Di Lazzaro et al. 2002) or increase overall corticospinal excitability (Maeda et al. 2000a, 2000b). However, it is unclear how these aftereffects depend on the variables selected for rTMS.
The effect of the number of conditioning stimuli has been recently investigated by Peinemann et al. (2004) who have shown that prolonged subthreshold conditioning (1800 pulses) increased corticospinal excitability for at least 30 minutes after the end of rTMS, whereas 150 subthreshold stimuli failed to facilitate the MEP amplitude. Following up on previous studies of low-frequency (1 Hz) rTMS, we investigated how the facilitatory effect of high-frequency (5 Hz) rTMS on corticospinal excitability depends on the intensity and duration of rTMS. A second aim of this study was to clarify whether cortical or spinal mechanisms contribute to the rTMS-induced increase in overall corticospinal excitability.
Materials and methods
Experimental approach
The experimental design is illustrated in Fig. 1. In three sets of experiments, we gave 5 Hz rTMS to the left M1-HAND and quantified distinct aspects of corticomotor excitability before, during, and after 5 Hz rTMS conditioning. All experiments were carried out on ten healthy young volunteers (six men and four women), each with no history of any neuropsychiatric disorder. The mean age was 28 years (range 21–40 years). All participants were consistent right-handers, according to the Edinburgh Handedness Inventory (Oldfield 1971). Written informed consent was obtained from all subjects prior to the study. The experiments were approved by the local ethics committee.
Experimental design. A In the first experiment, we gave 5 Hz rTMS to the left primary motor hand area (M1-HAND). In two separate sessions, rTMS was applied at 90% of active motor threshold (AMT) or 90% of resting motor threshold (RMT). Each rTMS session was divided into five blocks of 300 stimuli separated by an inter-train interval of approximately two minutes. Before and after rTMS conditioning, AMT, RMT, the amplitude of motor evoked responses (MEPs), and the duration of the cortical silent period (CSP) were determined in the right first dorsal interosseus (FDI) muscle. In addition, we also recorded 20 consecutive MEPs at rest between each rTMS train. B In the second experiment, we measured the mean MEP amplitude before and after 600 stimuli as well as after 1500 stimuli of 5 Hz rTMS. Stimulus intensity of rTMS was set at 90% of RMT. In three different sessions, either transcranial magnetic stimulation (TMS), anodal transcranial electrical stimulation (TES), or cervico-medullary stimulation (CMS) were used to elicit MEPs in the pre-activated FDI muscle. C In the third experiment, short-latency intracortical inhibition (SICI) and short-latency intracortical facilitation (SICF) were assessed before and after 600 stimuli rTMS conditioning. 5 Hz rTMS was given in two blocks of 300 stimuli. Stimulus intensity of rTMS was set at 90% of RMT
rTMS conditioning
Focal rTMS was given through a standard figure-of-eight shaped coil connected to a Magstim Rapid stimulator (Magstim Company, Whitland, Dyfed, UK). The mean loop diameters of the coil were 9 cm. The magnetic stimulus had a biphasic waveform with a pulse width of approximately 300 μs. The handle of the coil pointed backwards and laterally at a 45° angle away from the midline, approximately perpendicular to the line of the central sulcus. During the first phase of the biphasic stimulus, the current in the centre of the coil flowed toward the handle and induced a posterior-anterior current in the brain. The coil was placed tangentially to the scalp at the optimum scalp position to elicit MEPs in the contralateral first dorsal interosseus (FDI) muscle. We determined the optimal position for activation of the right FDI muscle by moving the coil in 0.5 cm steps around the presumed M1-HAND. The coil position at which a single stimulus at slightly suprathreshold intensity produced the largest MEPs in the right FDI muscle was marked with a pen as the “hot spot”. The rTMS protocols were in accordance with published safety recommendations (Wassermann 1998). EMG activity of the right FDI muscle was continuously monitored through loudspeakers throughout the entire rTMS session.
Experiment 1: Influence of conditioning effects on MEP amplitude and the duration of CSP
All participants underwent two sessions of 5 Hz rTMS (Fig. 1A). The intensity of rTMS was set at 90% resting motor threshold (RMT) or active motor threshold (AMT). In both sessions, participants received five rTMS trains of 300 pulses, each separated by approximately two minutes. The two rTMS sessions were given in a counterbalanced order at least a week apart.
Before and after rTMS, we applied single-pulse TMS over the conditioned M1-HAND to probe the effects of rTMS on corticospinal excitability using a high-power Magstim 200 stimulator (Magstim, Whitland, Dyfed) and a standard figure-of-eight coil, with external loop diameters of 9 cm. An identical coil position was used as for rTMS. The coil was positioned over the “hot spot” for stimulation of the contralateral FDI muscle and the handle of the coil pointed 45° postero-laterally. In contrast to rTMS, the magnetic stimuli had a monophasic pulse configuration. The monophasic magnetic stimulus had a rise-time of approximately 100 μs, decaying back to zero over approximately 0.8 ms. The coil current during the rising phase of the magnetic field flowed toward the handle. Thus, the induced current in the cortex flowed in an anterior-posterior direction. Motor evoked potentials (MEPs) were recorded from the right FDI muscle.
Measurements of corticospinal excitability were carried out in blocks. In each block, we measured the RMT, AMT, the MEP size at rest and the cortical silent period (CSP) in a fixed order (Fig. 1A). Each block of measurements started with an estimation of the RMT, which was defined as the minimum intensity that could evoke a peak-to-peak MEP of 50 μV in at least five out of ten consecutive trials in the relaxed FDI muscle. AMT was then assessed during voluntary tonic contraction at 5–10% of maximum force. The minimum intensity which produced at least three MEPs of 100 μV peak-to-peak amplitude in three out of five trials was defined as AMT.
After measurements of RMT and AMT, we gave suprathreshold single-pulse TMS at an intensity of 125% of RMT every five seconds. 20 consecutive MEPs were recorded from the right FDI muscle at rest and then during tonic isometric contraction. The peak-to-peak amplitude of each MEP was measured off-line and the mean MEP amplitude was calculated at rest and during contraction. The trials obtained during tonic contraction were also used to define the duration of the CSP, which was defined as the interval from stimulus delivery to the recurrence of voluntary activity (50% of baseline levels).
In addition, we assessed corticospinal excitability during the rTMS session. To this end, we recorded twenty consecutive MEPs at the end of each rTMS train using single-pulse TMS. Magnetic pulses were given at an intensity of 125% of RMT every five seconds. The mean peak-to-peak MEP amplitude was taken as a measure of corticospinal excitability.
Experiment 2: Influence of conditioning effects on MEP amplitude during tonic contraction
In five participants, we explored whether prolonged 5 Hz rTMS produces a change in excitability at the cortical or spinal level. All participants received 1500 stimuli of 5 Hz rTMS to the left M1-HAND at 90% of RMT (Fig. 1B). The rTMS session consisted of five trains of 300 stimuli separated by an inter-train interval of approximately two minutes.
Before and after 5 Hz rTMS, we used TMS, anodal transcranial electrical stimulation (TES), or cervico-medullary electrical stimulation (CMS) to assess rTMS-induced changes in MEP amplitude. TMS, TES and CMS measurements were performed in three separate experiments at least a week apart (Fig. 1B). MEPs were elicited every five seconds while participants performed a tonic pre-activation of the FDI muscle at 15% of maximum force level. Ten consecutive MEPs were recorded at baseline as well as after the second (600 stimuli) and fifth train (1500 stimuli) of the rTMS session (Fig. 1B). Stimulus intensity of TMS and TES was set just above AMT, since, at this intensity, anodal TES stimulates corticospinal neurones directly (D-wave) without concomitant indirect (trans-synaptic) activation (I-waves) (Day et al. 1989; Rothwell 1997; Di Lazzaro et al. 1999).
The procedure for TMS of left M1-HAND was identical to that for experiment 1. Anodal TES of the left M1-HAND was performed using two 0.9 cm Ag-AgCl surface electrodes. The cathode was placed over the vertex and the anode 6–7 cm laterally to the vertex (Rossini et al. 1994). Single pulses of up to 150 V (time constant 100 μs) were delivered using a Digitimer D180A stimulator (Digitimer, Welwyn Garden City, UK).
Because anodal TES at weak intensity reveals changes that occur at subcortical levels but not necessarily at the level of the spinal cord, CMS was applied in the same subjects to directly stimulate the corticospinal motor projections at brainstem level. TES was performed using the method described by Ugawa (1991). Surface electrodes were positioned over each mastoid process on both sides of the inion, with the anode on the right and the cathode on the left. Electrical stimuli were delivered via a Digitimer D180 stimulator. The stimulus intensity was varied from 35 to 55% of maximum stimulator output.
Experiment 3: Influence of conditioning effects on paired-pulse cortical excitability
The third experiment used paired-pulse TMS to assess the aftereffects of 600 conditioning stimuli on short-latency intracortical inhibition (SICI) and short-latency intracortical facilitation (SICF) in the left M1-HAND. Each rTMS session consisted of two 300-pulse trains given at 90% of RMT to the left M1-HAND (Fig. 1C).
Short-latency intracortical inhibition (SICI)
Changes in intracortical excitability of the stimulated left M1-HAND were examined with paired-pulse TMS in nine subjects. Paired-pulse excitability was measured before and after two 300-pulse trains of 5 Hz rTMS at 90% of RMT. We deliberately applied only two conditioning trains of 5 Hz rTMS (600 stimuli in total), since experiment 1 revealed that this rTMS protocol causes no consistent shifts in corticospinal excitability (as indexed by the unconditioned MEP amplitude). Therefore, any change in intracortical paired-pulse excitability was not confounded by concomitant changes in overall corticospinal excitability.
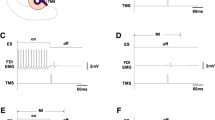
In a first block of measurements, we used the protocol introduced by Kujirai et al. (1993) to evaluate the magnitude of SICI at ISIs of 2 and 3 ms (Fig. 1C). The intensity of the conditioning stimulus (CS) was set at 80% of AMT. The intensity of the test stimulus (TS) was adjusted to elicit MEPs with peak-to-peak amplitudes of 0.5–1.0 mV. Paired-pulse TMS were intermingled with trials in which the TS was given alone. Ten trials were recorded for each condition.
Short latency intracortical facilitation (SICF)
In a second block of measurements, we used the paired-pulse paradigm introduced by Ziemann et al. (1998a) to explore facilitatory interactions between I-waves (Fig. 1C). In this paradigm, a suprathreshold stimulus (S1) is given 0.8–5.2 ms before a subthreshold stimulus (S2). Depending on the ISI, paired stimulation may produce a consistent increase in MEP amplitude compared to the MEP elicited by S1 alone. It is usually possible to separate three distinct “peaks” of MEP facilitation at ISIs around 1.1–1.5 ms, 2.5–2.9 ms, and 4.1–4.4 ms, which are separated by troughs. It is thought that MEP facilitation at each peak reflects a facilitatory interaction of cortical circuits that are also involved in the generation of distinct I-waves in response to transcranial cortex stimulation (Tokimura et al. 1996; Ziemann et al. 1998a, 1998b; Rothwell et al. 1999). Paired magnetic stimuli were applied over the “hot spot” for eliciting responses in the FDI muscle. The intensity of the first suprathreshold stimulus was adjusted to evoke a MEP of approximately 0.5 mV in the relaxed FDI muscle. The intensity of the second subthreshold stimulus was set at 90% of RMT. In two separate blocks, we evaluated 14 different ISIs (0.9, 1.1, 1.3, 1.5, 1.7, 1.9, 2.1, 2.3, 2.5, 2.7, 2.9, 3.1, 3.3, and 3.5 ms) to assess the first two “peaks” of SICF. Each block consisted of seven paired-pulse conditions at variable ISIs and a condition in which the first stimulus was given alone. Stimuli were delivered 5 s apart in a pseudo-random order. Ten trials were collected per condition (80 trials per block).
Data acquisition and analysis
MEPs were recorded from Ag-AgCl surface electrodes over the right FDI muscle using a belly-tendon montage. The signal was amplified and bandpass-filtered (32 Hz to 1 kHz) by a Digitimer D150 amplifier (Digitimer Ltd., Welwyn Garden City, UK) and stored at a sampling rate of 5 kHz on a personal computer for off-line analysis (SigAvg Software, Cambridge Electronic Design, Cambridge, UK). The EMG activity of the right FDI muscle was continuously monitored with visual (oscilloscope) and auditory (speakers) feedback throughout the entire experiment.
For MEPs evoked by single transcranial stimuli (experiments 1 and 2), peak-to-peak amplitudes were measured for each trial and averaged for each experimental condition, using NuCursor software (Sobell Research Dept. of Motor Neuroscience and Movement Disorders, Institute of Neurology, University College of London, United Kingdom). Time-dependent changes were assessed separately for each measure of excitability (motor threshold, peak-to-peak MEP amplitude at rest, duration of CSP), using a two-factorial analysis of variance (ANOVA) for repeated measurements. The ANOVA model included the factor intensity of rTMS (90% of AMT versus 90% of RMT) and time of measurement (before versus after 5 Hz rTMS). For the ANOVA that used motor threshold as the dependent variable, the factor motor state (rest versus contraction) was included as an additional factor. We used a two-factorial for repeated measurements to analyse the MEP changes in experiment 2. The ANOVA model included the factors type of transcranial stimulation (single-pulse TMS versus TES versus CMS) and time of measurement (before versus after 5 Hz rTMS).
For measurements involving paired-pulse TMS (experiment 3), we measured the peak to-peak MEP amplitude for each sweep and calculated the mean MEP amplitude for each experimental condition. Only sweeps without any voluntary EMG activity (>95% of all trials) were used for data analysis. The average peak-to-peak amplitudes of the conditioned MEPs at each ISI were then normalised to the mean MEP amplitude evoked by the suprathreshold stimulus alone (test condition). For SICF, we calculated the average of the normalised MEP amplitudes for ISIs ranging from 1.3 to 1.9 ms and from 2.1 to 2.9 ms in order to quantify SICF for the first and second peak respectively (Ziemann et al. 1998a). The normalized MEP amplitude was used as the dependent variable and entered in a two-factorial ANOVA for repeated measurements. The ANOVA model included the factors time of measurement (before versus after rTMS), and ISI (for SICI: ICIs of 2 and 3 ms; for SICF: ISIs ranging from 1.3 to 1.9 ms and from 2.1 to 2.9 ms).
The Greenhouse-Geisser method was used if necessary to correct for non-sphericity. Conditional on a significant F value, we performed post hoc comparisons using the Tuckey honest significant difference (HSD) test to directly compare the experimental conditions. We also computed a correlational analysis between changes in the magnitude of SICI and SICF using the Spearman Rank-correlation coefficient. For all analyses, a p-value of <0.05 was considered significant. All data are given as mean ±SE.
Results
No subject experienced any noticeable adverse effects from the rTMS procedure.
Experiment 1: Influence of conditioning effects on MEP amplitude and the duration of CSP
The mean intensity for rTMS at 90% of AMT was 37±3%, and for rTMS at 90% of RMT was 46±2%, expressed as percentage of maximum stimulator output. 5 Hz rTMS at 90% of RMT induced an increase in MEP amplitude at rest (Fig. 2A and Table 1), whereas rTMS at 90% of AMT did not cause any change in resting MEP amplitude (Fig. 2B and Table 1). Accordingly, the ANOVA which compared the conditioning effects of 5 Hz rTMS at 90% of AMT and 90% of RMT revealed a significant interaction between the factors time of measurement and intensity of stimulation (F(5,45)=13.44; p<0.001). This interaction was caused by a gradual increase in MEP amplitude during the course of 5 Hz rTMS at 90% of RMT, but not at 90% of AMT (Fig. 2). For 5 Hz rTMS at 90% of RMT, post hoc pairwise comparisons between MEP measurements at baseline and after each 300-pulse train of rTMS demonstrated a significant increase in MEP amplitude after 900 stimuli (p=0.001), 1200 stimuli (p<0.001), and 1500 stimuli (p<0.001). No significant changes in MEP size were found after 300 or 600 stimuli of 5 Hz rTMS (p=0.9 and p=0.17 respectively).
Change in mean MEP amplitudes at rest following subthreshold 5 Hz rTMS. Data represent the mean MEP amplitudes (±SEM) for ten subjects. MEPs were elicited at rest immediately after the end of each rTMS train (after 300, 600, 900, 1200, and 1500 conditioning stimuli). rTMS was given at 90% of RMT (A) or AMT (B ). The MEP size is expressed as percentage of the MEP size at baseline. The dotted line indicates the size of the unconditioned response (=100%). The asterisks indicate a significant difference in MEP amplitude relative to the baseline (paired-sample t-test; p<0.05)
For motor thresholds, ANOVA revealed a main effect of motor state (F(1,9)=46.3; p<0.002), because pre-activation always caused a consistent decrease in motor threshold relative to the RMT. Moreover, there was no main effect of the intensity of rTMS, or time of measurement, and no significant interaction between the three factors. This indicates that 5 Hz rTMS had no consistent effects on AMT and RMT regardless of the intensity of stimulation (Table 1). For the duration of the CSP, ANOVA demonstrated no significant main effect of the factors time of measurement and intensity of stimulation, nor any significant interaction between these factors, indicating that the duration of CSP was also unchanged after 5 Hz rTMS (Table 1).
Experiment 2: Influence of conditioning effects on MEP amplitude during tonic contraction
All types of low-intensity stimulation of M1 (TMS, TES and cervico-medullary stimulation) revealed a consistent increase in MEP amplitude in the isometrically contracting FDI muscle after 1500 stimuli at 90% of RMT (Fig. 3). This was confirmed by the ANOVA showing a significant main effect for the factor time (F(2,8)=35.4; p=0.0001) without an interaction between time and type of stimulation.
Changes in mean MEP amplitudes in the pre-activated muscle after 5 Hz rTMS. The mean amplitudes of MEPs elicited by TMS, TES and cervico-medullary stimulation (CMS) were measured at baseline as well as after 600 and 1500 conditioning stimuli at 90% of RMT. MEPs were recorded in the pre-activated right FDI muscle. Data represent the mean MEP amplitudes (±SEM) for five subjects. The asterisks indicate a significant increase in MEP amplitude after 5 Hz rTMS compared with baseline (paired-sample t-test; p<0.05)
Post hoc comparisons demonstrated that, after 1500 stimuli, the mean MEP amplitude in the contracting FDI increased significantly compared with baseline values (TMS: p=0.016; TES: p=0.018; CMS: p=0.003). By contrast, there was no change in MEP amplitude after 600 conditioning stimuli (TES: p=0.19; TMS: p=0.9; CMS: p=0.9) (Fig. 3).
Experiment 3: Influence of conditioning effects on paired-pulse cortical excitability
Short-latency intracortical inhibition (SICI)
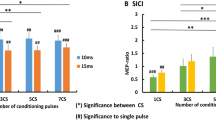
600 stimuli of 5 Hz rTMS at 90% of RMT led to a consistent decrease in SICI which was comparable in magnitude for inhibition at an ISI of 2 and 3 ms (Fig. 4). Accordingly, ANOVA showed a main effect of time of measurement (F(1,8)=29.9; p<0.0006), but no interaction between time of measurement and ISI. 600 stimuli of 5 Hz rTMS at 90% of RMT had no effect on the amplitude of the unconditioned MEP.
Effect of 5 Hz rTMS at 90% of RMT on short-latency intracortical inhibition (SICI). A The bar graph illustrates the mean magnitude of SICI at inter-stimulus intervals of 2–3 ms before and after 600 conditioning stimuli of nine healthy subjects. Each bar represents the normalized MEP amplitudes ±SEM at baseline (white bar) and after rTMS (black bar). The asterisks indicate a significant decrease in SICI after rTMS conditioning (paired-sample t-test; p<0.05). B The right panel illustrates the decrease in SICI at an ISI of 2 and 3 ms in a representative subject. Each trace represents the average waveform of ten MEPs
Intracortical facilitation at short intervals (SICF)
For statistical analysis, ISIs were grouped together to quantify mean facilitation during the first peak (ISIs ranging from 0.9 to 1.3 ms) and second peak (ISIs ranging from 2.5 to 2.9 ms) of SICF. 600 stimuli at 5 Hz and 90% of RMT caused a selective increase in SICF during the first but not during the second peak (Fig. 5A). This was reflected by a significant interaction between time of measurement and the ISI (F(1,8)=18.2; p=0.004). There was no effect of rTMS conditioning on the amplitude of the unconditioned MEP. Post hoc comparisons confirmed that the mean facilitation of the first peak was significantly greater after the conditioning rTMS session relative to the baseline (p<0.004; Fig. 5B). In contrast, the second peak was not significantly altered after rTMS. There was no significant correlation between the decrease in SICI at 2 and 3 ms and the increase in the first peak of SICF (p=0.4).
Conditioning effect of 5 Hz rTMS on short-latency intracortical facilitation (SICF). A The upper left panel illustrates the amount of SICF for each inter-stimulus interval. Data represent mean values before (white diamonds) and after (black squares) 600 stimuli of 5 Hz rTMS at 90% RMT. Data are expressed as percentages of the MEP amplitude elicited by the first stimulus alone. Error bars equal SEM. B The lower left panel shows the mean magnitudes of SICF for the first two peaks of SICF at baseline (white column) and after 600 stimuli of 5 Hz rTMS (black column). MEPs at ISIs from 1.1 to 1.5 ms and 2.5 to 2.9 ms were grouped to quantify the first and second peak of SICF respectively. The asterisks indicate a significant increase in the first peak of SICF after 5 Hz rTMS relative to baseline (paired-sample t-test; p<0.05). C The right panel illustrates SICF at ISIs between 0.9 and 3.5 ms in a representative subject. Each trace gives the average of ten MEPs recorded from the relaxed right FDI muscle at a given ISI
Discussion
The present study demonstrated that subthreshold 5 Hz rTMS to the human M1-HAND can cause a complex pattern of changes in excitability directly in the stimulated M1 and further downstream in the spinal cord. These conditioning effects critically depend on the intensity and duration of rTMS. When shorter periods of rTMS (in other words, 600 stimuli) were given to the M1 at 90% of RMT, rTMS conditioning induced distinct changes in two intracortical circuits generating SICI and SICF. Longer periods of 5 Hz rTMS (900–1500 stimuli) at 90% of RMT led to an increase in the overall level of corticospinal excitability, as indexed by an increase in mean MEP amplitude at rest. When low-intensity anodal TES or cervico-medullary electrical stimulation were used to elicit a MEP during voluntary contraction, MEPs were consistently facilitated after 1500 but not after 600 conditioning stimuli, indicating an increase in spinal excitability after prolonged 5 Hz rTMS. No MEP facilitation could be observed when prolonged rTMS was given at 90% of AMT. This complex pattern of excitability changes needs to be taken into account in studies that use focal rTMS to the M1-HAND to provoke acute reorganization in the intact human motor system. The implications of these data, in terms of our understanding of rTMS effects, are discussed in three sections: after effects of subthreshold 5 Hz rTMS on corticospinal, motor cortical, and spinal excitability.
Changes in overall corticospinal excitability
A consistent increase in corticospinal excitability (as indexed by an increase in the unconditioned MEP amplitude) was only produced if longer periods of subthreshold 5 Hz rTMS (≥900 stimuli) were given to the left M1-HAND at 90% of RMT. Upon reducing the intensity of AMT to 90%, prolonged 5 Hz rTMS failed to produce a lasting effect on corticospinal excitability. This implies that longer periods of 5 Hz rTMS need to be given at a rather high intensity (close to RMT) in order to induce a consistent increase in corticospinal excitability. A similar picture has emerged for the suppressive aftereffect of low-frequency (1 Hz ) rTMS to the M1 on corticospinal excitability. Longer periods of 1 Hz rTMS at intensities just below RMT led to an enduring attenuation of corticospinal excitability, whereas short periods of rTMS failed to produce a suppressive effect (Touge et al. 2001). In analogy to the aftereffects of 5 Hz rTMS in this study, longer periods of 1 Hz rTMS over M1 were insufficient to induce a decrease in corticospinal excitability, if the intensity was reduced to 90% of AMT (Gerschlager et al. 2001).
There are at least two explanations why rTMS needs to be given at a relatively high intensity in order to provoke a lasting change in corticospinal excitability. First, epidural recordings from the cervical cord have shown that the threshold for evoking descending volleys in the corticospinal tract corresponds to AMT (Di Lazzaro et al. 1998, 1999). If repetitive postsynaptic stimulation of the corticospinal output neurons is a prerequisite to altering corticospinal excitability, only rTMS at intensities above AMT will be capable of shaping corticospinal excitability.
Second, when rTMS is applied 2.5–3 cm rostral to the optimal site for M1-HAND stimulation, prolonged 1 Hz or 5 Hz rTMS of the dorsal premotor cortex (PMd) can produce a lasting decrease or increase in corticospinal excitability, respectively (Gerschlager et al. 2001; Rizzo et al. 2004). Importantly, the conditioning effects of premotor rTMS on corticospinal excitability already occur at a very low intensity of 90% AMT (Gerschlager et al. 2001; Rizzo et al. 2004). Since rTMS over the M1-HAND exhibits a higher threshold for inducing a change in corticospinal excitability than ipsilateral PMd, it has been proposed that the aftereffects on corticospinal excitability are caused by repetitive stimulation of premotor-to-motor connections rather than by direct stimulation of neurones in the M1 itself (Gerschlager et al. 2001). According to this hypothesis, only rTMS at higher stimulus intensities would cause a sufficient spread of excitation from the M1 site to the adjacent premotor cortex to induce an effective stimulation of premotor-to-motor connections.
In addition to stimulus intensity, the number of stimuli had an impact on the conditioning effects of rTMS, showing a gradual build-up of MEP facilitation during the course of rTMS. At an intensity of 90% of RMT, 300 and 600 stimuli failed to produce a significant MEP. This implies that longer periods of 5 Hz rTMS are necessary to enhance corticospinal excitability. A similar pattern has also been reported for subthreshold 1 Hz rTMS of the M1. Mirroring the facilitatory effect of 5 Hz rTMS in this study, the suppression of corticospinal excitability gradually increased with the number of conditioning stimuli per session (Touge et al. 2001). For rTMS to the M1-HAND, the general rule that seems to be emerging is that the strongest and longest aftereffects on corticospinal excitability are produced by longer periods of rTMS (≥1500 stimuli) and at higher intensities (just below RMT).
Touge et al. (2001) reported that 1500 stimuli of 1 Hz rTMS produced a lasting decrease in corticospinal excitability in the relaxed, but not in the actively contracting, FDI muscle. They argued that the lack of any conditioning effect on MEPs evoked during contraction is not consistent with the idea that rTMS depresses transmission in synaptic connections to pyramidal cells activated by the test TMS pulse. In contrast to 1 Hz rTMS conditioning, we found that prolonged 5 Hz rTMS at 90% of RMT facilitated MEPs in the relaxed and tonically contracting FDI muscle. The enhancement of corticospinal excitability at rest and during voluntary pre-activation supports the notion that prolonged 5 Hz rTMS can enhance synaptic transmission in the corticospinal output system. However, a lasting increase in MEP amplitudes at rest and during contraction may be caused by a potentiation of synaptic connections on pyramidal cells in the M1 or to a potentiation of synaptic connections between pyramidal cells and spinal motoneurones in the cervical spinal cord. As outlined below, the present results show that 5 Hz rTMS can produce a lasting potentiation of synaptic transmission in both the M1 and the spinal cord.
Changes in motor cortical excitability
Short-latency intracortical inhibition
In the third experiment, 600 stimuli of subthreshold rTMS attenuated SICI without affecting corticospinal excitability. This is in good agreement with a recent study by Di Lazzaro et al. (2002) who reported a transient decrease in SICI following a short train of subthreshold 5 Hz rTMS. In addition to MEP measurments, Di Lazzaro et al. (2002) performed epidural recordings of the descending corticospinal volleys to show that the decrease in SICI was in fact due to a change in excitability at the cortical level. Since the SICI reflects the excitability of intracortical GABAergic circuits (Ziemann et al. 1996; for a review, see Ziemann 1999), we infer that short periods of subthreshold 5 Hz rTMS of the M1 provides a means of reducing short-lasting intracortical inhibition in the stimulated M1.
Cortical silent period
We also assessed changes in the duration of the CSP after rTMS conditioning. In contrast to SICI, which is thought to reflect the excitability of inhibitory neurons subserving short-lasting, presumably GABAA-ergic inhibition, the duration of the CSP depends upon the excitability of a different set of intracortical inhibitory interneurons mediating long-lasting, presumably GABAB-ergic inhibition (Hallett et al. 2000). In this study, subthreshold 5 Hz rTMS failed to alter the duration of the CSP, but reduced the amount of SICI, indicating a different responsiveness of distinct inhibitory circuits in the M1 to 5 Hz rTMS conditioning.
In a recent study by Rizzo et al. (2004), a prolonged session of subthreshold 5 Hz rTMS to the PMd shortened the duration of CSP. Premotor 5 Hz rTMS also failed to modify SICI, but induced a decrease in paired-pulse excitability at an intermediate ISI of 7 ms (Rizzo et al. 2004). The observation that 5 Hz rTMS to the M1 and PMd induce different patterns of conditioning effects on intracortical inhibition in the M1 is at variance with the hypothesis that a spread of excitation to adjacent PMd accounts for the conditioning effects of rTMS to the M1-HAND.
Short latency intracortical facilitation (SICF)
Using the conditioning-test paradigm introduced by Ziemann et al. (1998a), a new finding of the present study was that 600 stimuli of 5 Hz rTMS at 90% of RMT increased the first peak of SICF. This first peak at ISIs of 1.1–1.5 ms is thought to reflect the excitability of intracortical circuits generating the first I-wave in the corticospinal tract in response to transcranial stimulation (for a review, see Rothwell 1999). By contrast, the second peak at ISIs of 2.5–2.9 ms, which can be also mediated by spinal mechanisms, was not altered by 5 Hz rTMS, supporting the notion that the first and second peaks reflect the excitability of distinct circuits.
The facilitatory effect on the first peak of SICF indicates that subthreshold 5 Hz rTMS can potentiate the efficacy of synaptic connections projecting on pyramidal neurones in the stimulated M1-HAND. The increase in SICF did not correlate with the decrease in SICI, lending further support to the notion that subthreshold 5 Hz rTMS can modify separate subpopulations of intracortical neurons. It is worth noting that both conditioning effects—the decrease in SICI and the increase in SICF—shifted the balance of intracortical excitability towards a stronger facilitation. This augmentation of intracortical facilitation might provide a clue to explaining the increase in resting regional glucose metabolism (Siebner et al. 2000) and regional cerebral blood flow in the M1 (Siebner et al. 2001; Rounis et al. 2003) following subthreshold 5 Hz rTMS. In the context of an enhanced intracortical facilitation, any synaptic input to the M1 will cause a stronger synaptic activity in the M1, resulting in an overall increase in regional synaptic activity (as indexed by an increase in regional glucose metabolism or blood flow).
Changes in spinal excitability
It is important to recall that longer periods of 5 Hz rTMS only caused an increase in corticospinal excitability when rTMS was given at 90% RMT, not at 90% of AMT. Since the effective intensity (90% of RMT) was above the threshold for evoking descending volleys in corticospinal neurones (Di Lazzaro et al. 1999), it is possible that repetitive transsynaptic stimulation of spinal motoneurons caused a lasting increase in excitability at spinal cord level. To explore the spinal contribution to the overall increase in corticospinal excitability, we applied monophasic TMS and anodal TES at intensities just above AMT to the left M1 and recorded the MEPs in the tonically contracting FDI muscle.
Epidural recordings of the spinal volleys elicited by single transcranial stimuli over the M1 have revealed that anodal TES given at AMT selectively evokes a D wave (Di Lazzaro et al. 1998). Since the D-wave results from direct stimulation of the corticospinal axons, the motor response elicited by anodal TES at an intensity just above AMT is not affected by a change in excitability in the M1 (Di Lazzaro et al. 1998). Therefore, any change in MEP amplitude elicited by TES at an intensity around AMT is thought to reflect a change in excitability at sub-cortical level but not necessarily at the level of the spinal cord (Rothwell 1997). Therefore, we also stimulated the corticospinal projections at the brain stem level (CMES) to assess the level of spinal excitability.
TMS and TES at an intensity just above AMT as well as CMES indicated a consistent facilitation of mean MEP amplitude in the tonically contracting FDI muscle after prolonged 5 Hz rTMS. This finding strongly suggests that the increase in overall corticospinal excitability after prolonged rTMS was associated with an increase in spinal excitability.
An augmentation of spinal excitability was only observed after 1500 stimuli, not after 600 stimuli. In contrast, the conditioning effects of subthreshold 5 Hz rTMS on intracortical paired-pulse excitability occur after a relatively small number of conditioning stimuli.
In conclusion, our data suggest that short periods of rTMS over M1 tend to provoke spatially-restricted effects on intracortical interneurones in the stimulated cortex, whereas longer periods of 5 Hz rTMS can produce conditioning effects on excitability levels in distant motor areas (spinal cord).
References
Chen R, Classen J, Gerloff G, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low frequency transcranial magnetic stimulation. Neurology 48:1398–1403
Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD (1989) Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412:449–473
Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC (1998) Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroen Clin Neuro 109:397–401
Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC (1999) Direct recordings of descending volleys after transcranial magnetic and electric motor cortex stimulation in conscious humans. Electroen Clin Neuro Suppl 51:120–126
Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC (2002) Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res 147:108–113
Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J (2002) Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol 113:1136–1141
Gangitano M, Valero-Cabre A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A (2002) Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol 113:1249–1257
Gerschlager W, Siebner HR, Rothwell JC (2001) Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 57:379–380
Hallett M (2000) Transcranial magnetic stimulation and the human brain. Nature 6792:147–150
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A (2000a) Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111:800–805
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A (2000b) Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133:425–430
Modugno N, Nakamura Y, MacKinnon CD, Filipovic SR, Bestmann S, Berardelli A, Rothwell JC (2001) Motor cortex excitability following short trains of repetitive magnetic stimuli. Exp Brain Res 140:453–459
Muellbacher W, Ziemann U, Boroojerdi B, Hallett M (2000) Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111:1002–1007
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117:847–858
Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD (1998) Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15:333–343
Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR (2000) Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett 296:21–24
Peinemann A, Reimer B, Löer C, Quartarone A, Münchau A, Conrad B, Siebner HR (2004) Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the motor cortex. Clin Neurophysiol 115(7):1519–26
Rizzo V, Siebner H, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC (2004) Shaping the excitability of human motor cortex with premotor rTMS. J Physiol 554:483–495
Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A (2002) Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol 113:101–107
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroen Clin Neuro 91:79–92
Rothwell JC (1997) Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Meth 74:113–122
Rothwell JC (1999) Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. Electroen Clin Neuro Suppl 51:113–119
Rounis E, Siebner HR, et al (2003) The effect of 5 Hz repetitive transcranial magnetic stimulation over the left primary motor hand area on regional blood flow and motor cortex excitability. NeuroImage 19:17822 (available on CD-Rom in Issue 2, Suppl 1)
Siebner HR, Rothwell J (2003) Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148:1–16
Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P (2000) Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology 54:956–963
Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A (2001) Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. NeuroImage 14:883–890
Sommer M, Wu T, Tergau F, Paulus W (2002) Intra- and interindividual variability of motor responses to repetitive transcranial magnetic stimulation. Clin Neurophysiol 113:265–269
Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC (1996) Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroen Clin Neuro 101:263–272
Touge T, Gerschlager W, Brown P, Rothwell JC (2001) Are the after effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol 112:2138–2145
Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD (1991) Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol 29:418–27
Valero-Cabre A, Oliveri M, Gangitano M, Pascual-Leone A (2001) Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport 12:3845–3848
Wassermann EM (1998) Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroen Clin Neuro 108:1–16
Wu T, Sommer M, Tergau F, Paulus W (2000) Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett 287:37–40
Ziemann U (1999) Intracortical inhibition and facilitation in the conventional paired TMS paradigm. Electroen Clin Neuro Suppl 51:127–136
Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996) Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40:367–378
Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W (1998a) Demonstration of facilitatory I waves interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 511:181–190
Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W (1998b) Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroen Clin Neuro 109:321–330
Acknowledgements
H.R. Siebner was supported by the Bundesministerium für Bildung und Forschung (grant 01GO0206) and the Volkswagen Foundation (grant I/79 932).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quartarone, A., Bagnato, S., Rizzo, V. et al. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res 161, 114–124 (2005). https://doi.org/10.1007/s00221-004-2052-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2052-5