Abstract
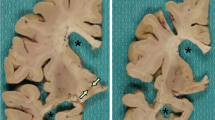
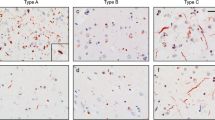
Frontotemporal lobar degeneration (FTLD) can be pathologically subdivided into tau-positive and tau-negative types. The most common tau-negative variant is FTLD with ubiquitin-immunoreactive lesions (FTLD-U). Recently, the TAR DNA binding protein 43 (TDP-43) was identified in neuronal inclusions in FTLD-U. After applying TDP-43 immunohistochemistry to a series of 44 cases of FTLD-U with no secondary pathology, three cases (7%) were identified with ubiquitin- and p62-positive neuronal cytoplasmic inclusions (NCI) that were negative for TDP-43. All the three cases had marked brain atrophy with striking atrophy of the striatum. Cases 1 and 2 presented at ages 43 and 38, respectively, as behavioral variant frontotemporal dementia (1 with positive family history) and had ubiquitin- and p62-positive NCI in frontotemporal neocortex and dentate granule cells of the hippocampus. Case 3 presented with the corticobasal syndrome. Unlike the other two cases, ubiquitin- and p62-positive NCI were also visible on hematoxylin and eosin stain. There were no neuronal intranuclear inclusions. Electron microscopic examination of the NCI in cases 2 and 3 revealed granulofilamentous inclusions. These cases confirm the existence of TDP-43-negative FTLD-U and extend the clinical and pathological spectrum of this disorder. The findings raise the possibly of an as yet identified protein that may play a pathogenic role in tau-negative FTLD.




Similar content being viewed by others
References
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445. doi:10.1002/ana.21154
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611. doi:10.1016/j.bbrc.2006.10.093
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Cairns NJ, Grossman M, Arnold SE, Burn DJ, Jaros E, Perry RH et al (2004) Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 63:1376–1384
Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Yen SH (1990) Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab Invest 63:87–99
Dickson DW, Wertkin A, Mattiace LA, Fier E, Kress Y, Davies P et al (1990) Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer’s disease. Acta Neuropathol 79:486–493. doi:10.1007/BF00296107
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. doi:10.1016/0022-3956(75)90026-6
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J et al (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001. doi:10.1093/hmg/ddl241
Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM et al (2008) Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115:133–145. doi:10.1007/s00401-007-0257-y
Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH et al (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99:663–672. doi:10.1007/s004010051177
Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T et al (2007) TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 130:1386–1394. doi:10.1093/brain/awm065
Holm IE, Englund E, Mackenzie IR, Johannsen P, Isaacs AM (2007) A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3. J Neuropathol Exp Neurol 66:884–891. doi:10.1097/nen.0b013e3181567f02
Josephs KA, Dickson DW (2007) Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging 28:1718–1722. doi:10.1016/j.neurobiolaging.2006.07.010
Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K et al (2004) Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 30:369–373. doi:10.1111/j.1365-2990.2003.00545.x
Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW (2006) Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol 63:506–512. doi:10.1001/archneur.63.4.506
Knopman DS, Mastri AR, Frey WH 2nd, Sung JH, Rustan T (1990) Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology 40:251–256
Kusaka H, Matsumoto S, Imai T (1990) An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol 80:660–665. doi:10.1007/BF00307636
Lee S, Park YD, Yen SH, Ksiezak-Reding H, Goldman JE, Dickson DW (1989) A study of infantile motor neuron disease with neurofilament and ubiquitin immunocytochemistry. Neuropediatrics 20:107–111
Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW (2003) Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol 162:213–218
Lipton AM, White CL 3rd, Bigio EH (2004) Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 108:379–385. doi:10.1007/s00401-004-0900-9
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH et al (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549. doi:10.1007/s00401-006-0138-9
Mackenzie IR, Foti D, Woulfe J, Hurwitz TA (2008) Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131:1282–1293. doi:10.1093/brain/awn061
Matsumoto S, Kusaka H, Murakami N, Hashizume Y, Okazaki H, Hirano A (1992) Basophilic inclusions in sporadic juvenile amyotrophic lateral sclerosis: an immunocytochemical and ultrastructural study. Acta Neuropathol 83:579–583. doi:10.1007/BF00299405
Munoz-Garcia D, Ludwin SK (1984) Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Ann Neurol 16:467–480. doi:10.1002/ana.410160408
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229. doi:10.1007/s00401-007-0261-2
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
Sasaki S, Toi S, Shirata A, Yamane K, Sakuma H, Iwata M (2001) Immunohistochemical and ultrastructural study of basophilic inclusions in adult-onset motor neuron disease. Acta Neuropathol 102:200–206
Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H et al (2005) Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37:806–808. doi:10.1038/ng1609
Yokota O, Tsuchiya K, Terada S, Ishizu H, Uchikado H, Ikeda M et al (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575. doi:10.1007/s00401-007-0329-z
Disclosure statement
The authors do not have any disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH grants P50-AG16574, P50-NS40256 and Pacific Alzheimer Research Foundation (PARF) grant C06-01.
Rights and permissions
About this article
Cite this article
Josephs, K.A., Lin, WL., Ahmed, Z. et al. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 116, 159–167 (2008). https://doi.org/10.1007/s00401-008-0397-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0397-8