Abstract
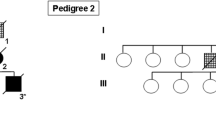
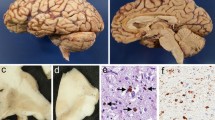
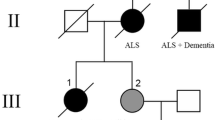
A number of recent studies have described cases with tau-positive globular oligodendroglial inclusions (GOIs) and such cases have overlapping pathological features with progressive supranuclear palsy (PSP), but present with clinical features of motor neuron disease (MND) and/or frontotemporal dementia (FTD). These two clinical phenotypes have been published independently and as a result, have come to be considered as distinct disease entities. We describe the clinicopathological and biochemical features of two cases with GOIs: one with clinical symptoms suggestive of MND and the other with FTD. Histological changes in our two cases were consistent with their clinical symptoms; the MND case had severe neurodegeneration in the primary motor cortex and corticospinal tract, whereas the FTD case had severe involvement of the frontotemporal cortices and associated white matter. Immunohistochemistry in both cases revealed significant 4-repeat (4R) tau pathology primarily in the form of GOIs, but also in astrocytes and neurons. Astrocytic tau pathology was morphologically similar to that seen in PSP, but in contrast was consistently negative for Gallyas silver staining. Tau-specific western blotting revealed 68, 64 and 35 kDa bands, showing further overlap with PSP. The underlying neuropathological features of these two cases were similar, with the major difference relating to the regional distribution of pathology and resulting clinical symptoms and signs. The globular nature of glial inclusions and the non-fibrillar properties of tau in astrocytes are characteristic features that allow them to be distinguished from PSP and other tauopathies. We, therefore, propose the term globular glial tauopathy as an encompassing term to classify this emerging class of 4R tauopathy.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AGD:
-
Argyrophillic grain disease
- ALS:
-
Amyotrophic lateral sclerosis
- CBD:
-
Corticobasal degeneration
- CBS:
-
Corticobasal syndrome
- CST:
-
Corticospinal tract
- FTD:
-
Frontotemporal dementia
- FTDP-17:
-
Frontotemporal dementia with parkinsonism linked to chromosome 17
- FTD-P-MND:
-
Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, parkinsonism, and motor neuron disease
- FTLD:
-
Frontotemporal lobar degeneration
- FUS:
-
Fused in sarcoma protein
- GGT:
-
Globular glial tauopathy
- GOIs:
-
Globular oligodendroglial inclusions
- IHC:
-
Immunohistochemistry
- MND:
-
Motor neuron disease
- MSA:
-
Multiple system atrophy
- MSTD:
-
Multiple system tauopathy with dementia
- NFT:
-
Neurofibrillary tangles
- PiD:
-
Pick’s disease
- PLS:
-
Primary lateral sclerosis
- PSP:
-
Progressive supranuclear palsy
- PSP-CST:
-
Atypical PSP with corticospinal tract degeneration
- TDP-43:
-
TAR DNA-binding protein 43
- WMT-GGI:
-
White matter tauopathy with globular glial inclusions
- 27 kP:
-
27,000×g pellet
- 3R:
-
3-repeat tau
- 4R:
-
4-repeat tau
References
Baker M, Litvan I, Houlden H et al (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8:711–715
Bergeron C, Morris HR, Rossor M (2003) Pick’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 124–131
Berry RW, Quinn B, Johnson N, Cochran EJ, Ghoshal N, Binder LI (2001) Pathological glial tau accumulations in neurodegenerative disease: review and case report. Neurochem Int 39:469–479
Bigio EH, Lipton AM, Yen SH et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(Suppl 2):II6–II15
Dickson DW, Litvan I (2003) Corticobasal degeneration. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 115–123
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Ferrer I, Hernandez I, Boada M et al (2003) Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol 106:419–435
Fu YJ, Nishihira Y, Kuroda S et al (2010) Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32
Gallyas F (1971) Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19:1–8
Ghetti B, Hutton ML, Wszolek ZK (2003) Frontotemporal dementia and parkinsonism linked to chromosome 17 associated with tau gene mutations (FTDP-17T). In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 86–102
Hauw JJ, Daniel SE, Dickson D et al (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C (1990) Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119:182–186
Hauw J, Agid Y (2003) Progressive supranuclear palsy (PSP) or Steele-Richardson-Olszewski disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 103–114
Ikeda K, Akiyama H, Haga C, Kondo H, Arima K, Oda T (1994) Argyrophilic thread-like structure in corticobasal degeneration and supranuclear palsy. Neurosci Lett 174:157–159
Josephs KA, Katsuse O, Beccano-Kelly DA et al (2006) Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65:396–405
Komori T (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 9:663–679
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975
Lantos PL, Quinn N (2003) Multiple system atrophy. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 203–214
Lashley T, Rohrer J, Bandopadhyay R et al (2011) A comparative clinical, pathological and biochemical study of FUS proteinopathies. Brain (in press)
Litvan I, Hauw JJ, Bartko JJ et al (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Mackenzie IR (2007) The neuropathology of FTD associated With ALS. Alzheimer Dis Assoc Disord 21:S44–S49
Mackenzie IR, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Ohara S, Tsuyuzaki J, Oide T et al (2002) A clinical and neuropathological study of an unusual case of sporadic tauopathy. A variant of corticobasal degeneration? Neurosci Lett 330:84–88
Oyanagi K, Wada M (1999) Neuropathology of parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 246(Suppl 2):II19–II27
Poorkaj P, Bird TD, Wijsman E et al (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825
Powers JM, Byrne NP, Ito M et al (2003) A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol 106:181–187
Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC (1992) Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 115(Pt 2):495–520
Sakai K, Piao YS, Kikugawa K et al (2006) Corticobasal degeneration with focal, massive tau accumulation in the subcortical white matter astrocytes. Acta Neuropathol 112:341–348
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Swash M, Desai J, Misra VP (1999) What is primary lateral sclerosis? J Neurol Sci 170:5–10
Takahashi T, Amano N, Hanihara T et al (1996) Corticobasal degeneration: widespread argentophilic threads and glia in addition to neurofibrillary tangles. Similarities of cytoskeletal abnormalities in corticobasal degeneration and progressive supranuclear palsy. J Neurol Sci 138:66–77
Tan CF, Piao YS, Kakita A et al (2005) Frontotemporal dementia with co-occurrence of astrocytic plaques and tufted astrocytes, and severe degeneration of the cerebral white matter: a variant of corticobasal degeneration? Acta Neuropathol 109:329–338
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Wray S, Saxton M, Anderton BH, Hanger DP (2008) Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J Neurochem 105:2343–2352
Acknowledgments
Professor Revesz, Dr Holton and Dr Ahmed are supported by the Multiple System Atrophy Trust (formerly known as the Sarah Matheson Trust for Multiple System Atrophy). Dr Silveira-Moriyama, Dr Holton, Dr de Silva and Dr Doherty are supported by the Reta Lila Weston Trust for Medical Research. Dr de Silva is funded by grants by the Medical Research Council (G0501560) and Cure PSP+. Part of this work was undertaken at UCLH/UCL, who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Z. Ahmed and K. Doherty contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Ahmed, Z., Doherty, K.M., Silveira-Moriyama, L. et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122, 415–428 (2011). https://doi.org/10.1007/s00401-011-0857-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0857-4