Abstract
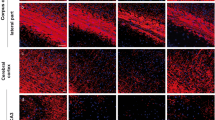
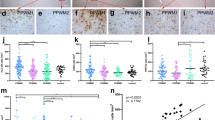
Oligodendrocyte gap junctions (GJs) are vital for central nervous system myelination, but their involvement in multiple sclerosis (MS) pathology remains unknown. The aim of this study was to examine alterations of oligodendrocyte and related astrocyte GJs in MS lesions and normal-appearing white matter (NAWM). Post-mortem brain samples from 9 MS and 11 age-matched non-MS control patients were studied. Tissue sections that included both chronic active and inactive lesions were characterized neuropathologically with Luxol Fast Blue staining and immunostaining for myelin oligodendrocyte glycoprotein (MOG) and the microglial marker Iba1. We analyzed the expression of Cx32 and Cx47 in oligodendrocytes and of Cx43, the major astrocytic partner in oligodendrocyte–astrocyte (O/A) GJs by quantitative immunoblot and real-time PCR. Formation of GJ plaques was quantified by immunohistochemistry. Compared to control brains, both Cx32 and Cx47 GJ plaques and protein levels were reduced in and around MS lesions, while Cx43 was increased as part of astrogliosis. In the NAWM, Cx32 was significantly reduced along myelinated fibers whereas Cx47 showed increased expression mainly in oligodendrocyte precursor cells (OPCs). However, OPCs showed only limited connectivity to astrocytes. Cx43 showed modestly increased levels in MS NAWM compared to controls, while GJ plaque counts were unchanged. Our findings indicate that oligodendrocyte GJs are affected not only in chronic MS lesions but also in NAWM, where disruption of Cx32 GJs in myelinated fibers may impair myelin structure and function. Moreover, limited O/A GJ connectivity of recruited OPCs in the setting of persistent inflammation and astrogliosis may prevent differentiation and remyelination.






Similar content being viewed by others
References
Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul J, Haydon P, Paul D, Huang Y, Abrams C, Scherer S (2008) Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res 86:992–1006
Altevogt BM, Paul DL (2004) Four classes of intercellular channels between glial cells in the CNS. J Neurosci 24:4313–4323
Belliveau DJ, Kidder GM, Vaus CCG (1991) Expression of gap junction genes during postnatal neural development. Dev Genet 12:308–317
Brand-Schieber E, Werner P, Iacobas DA, Iacobas S, Beelitz M, Lowery SL, Spray DC, Scemes E (2005) Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J Neurosci Res 80:798–808
Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H (1995) Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 38:788–796
Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–173
Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH (2001) Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 56:926–933
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517
Filippi M, Tortorella C, Bozzali M (1999) Normal-appearing white matter changes in multiple sclerosis: the contribution of magnetic resonance techniques. Mult Scler 5:273–282
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132:1175–1189
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 354:942–955
Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:87–99
Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L (2000) Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int 37:317–329
Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R (2010) Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol 69:1017–1033
Kamasawa N, Sik A, Morita M, Yasumura T, Davidson K, Nagy J, Rash J (2005) Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience 136:65–86
Kielian T (2008) Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem 106(3):1000–1016
Kleopa KA, Yum SW, Scherer SS (2002) Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J Neurosci Res 68:522–534
Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS (2004) Unique distribution of gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia 47:346–357
Kunze A, Congreso MR, Hartmann C, Wallraff-Beck A, Huttmann K, Bedner P, Requardt R, Seifert G, Redecker C, Willecke K, Hoffmann A, Pfeifer A, Theis M, Steinhauser C (2009) Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 106:11336–11341
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Lassmann H, Raine CS, Antel J, Prineas JW (1998) Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol 86:213–217
Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H (1996) Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol 6:259–274
Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF (2009) Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29:7743–7752
Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H (2010) Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 58:1104–1117
Magnotti LM, Goodenough DA, Paul DL (2011) Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia 59:1064–1074
Melanson-Drapeau L, Beyko S, Dave S, Hebb A, Franks D, Sellitto C, Paul D, Bennett S (2003) Oligodendrocyte progenitor enrichment in the connexin32 null-mutant mouse. J Neurosci 23:1759–1768
Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2003) Connexins are critical for normal myelination in the CNS. J Neurosci 23:5963–5973
Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2006) Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci 26(48):10984–10991
Nagy JI, Patel D, Ochalski PAY, Stelmack GL (1999) Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88(2):447–468
Nagy JI, Rash JE (2000) Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Rev 32(1):29–44
Nagy JI, Ionescu AV, Lynn BD, Rash JE (2003) Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: implications from normal and connexin32 knockout mice. Glia 44:205–218
Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K (2003) Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci 23:4549–4559
Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS (2007) Loss-of-function connexin47 mutations cause Pelizaeus–Merzbacher-like disease. Mol Cell Neurosci 34:629–641
Orthmann-Murphy JL, Salsano E, Abrams CK, Bizzi A, Uziel G, Freidin MM, Lamantea E, Zeviani M, Scherer SS, Pareyson D (2009) Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 132:426–438
Paulson HL, Garbern JY, Hoban TF, Krajewski KM, Lewis RA, Fischbeck KH, Grossman RI, Lenkinski R, Kamholz JA, Shy ME (2002) Transient central nervous system white matter abnormality in X-linked Charcot–Marie–Tooth disease. Ann Neurol 52:429–434
Paznekas WA, Boyadjiev SA, Shapiro R, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW (2003) Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 72:408–418
Pelletier D, Nelson SJ, Oh J, Antel JP, Kita M, Zamvil SS, Goodkin DE (2003) MRI lesion volume heterogeneity in primary progressive MS in relation with axonal damage and brain atrophy. J Neurol Neurosurg Psychiatry 74:950–952
Plumb J, McQuaid S, Mirakhur M, Kirk J (2002) Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol 12:154–169
Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak E, Hoffman B, Morgan BP (2001) Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 50:646–657
Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T (1997) Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a ‘‘panglial syncytium’’ that is not coupled to neurons. J Comp Neurol 388(2):265–292
Rash JE, Yasumura T, Dudek FE, Nagy JI (2001) Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci 21(6):1983–2000
Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27:13781–13792
Reynolds R, Dawson M, Polito A, Cenci di Bello I, Papadopoulos D, Pham-Dinh D, Levine J (2002) The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG–EAE and MS. J Neurocytol 31:523–536
Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O (2011) The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol 122:155–170
Roscoe WA, Messersmith E, Meyer-Franke A, Wipke B, Karlik SJ (2007) Connexin 43 gap junction proteins are up-regulated in remyelinating spinal cord. J Neurosci Res 85:945–953
Sargiannidou I, Ahn M, Enriquez AD, Peinado A, Reynolds R, Abrams CK, Scherer SS, Kleopa KA (2008) Human oligodendrocytes express Cx31.3: function and interactions with Cx32 mutants. Neurobiol Dis 30:221–233
Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA (2009) Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J Neurosci 29:4748–4761
Sharma R, Fischer MT, Bauer J, Felts PA, Smith KJ, Misu T, Fujihara K, Bradl M, Lassmann H (2010) Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol 120:223–236
Soffer D, Raine CS (1980) Morphologic analysis of axo-glial membrane specializations in the demyelinated central nervous system. Brain Res 186:301–313
Sutor B, Schmolke C, Teubner B, Schirmer C, Willecke K (2000) Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32-deficient mice. Cereb Cortex 10(7):684–697
Taylor RA, Simon EM, Marks HG, Scherer SS (2003) The CNS phenotype of X-linked Charcot–Marie–Tooth disease: more than a peripheral problem. Neurology 61:1475–1478
Toews JC, Schram V, Weerth SH, Mignery GA, Russell JT (2007) Signaling proteins in the axoglial apparatus of sciatic nerve nodes of Ranvier. Glia 55(2):202–213
Tress O, Maglione M, Zlomuzica A, May D, Dicke N, Degen J, Dere E, Kettenmann H, Hartmann D, Willecke K (2011) Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus–Merzbacher-like disease in humans. PLoS Genet 7:e1002146 (Epub 1002011 Jul 1002147)
Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J (2004) Mutations in the gene encoding gap junction protein alpha 12 (Connexin 46.6) cause Pelizaeus–Merzbacher-like disease. Am J Hum Genet 75:251–260
Vavlitou N, Sargiannidou I, Markoullis K, Kyriacou K, Scherer SS, Kleopa KA (2010) Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy. J Neuropathol Exp Neurol 69:945–958
Véga C, Martiel JL, Drouhault D, Burckhart MF, Coles JA (2003) Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol 546:551–564
Venance L, Cordier J, Monge M, Zalc B, Glowinski J, Giame C (1995) Homotypic and heterotypic coupling mediated by gap junctions during glial cell differentiation in vitro. Eur J Neurosci 7:451–461
Von Blankenfeld G, Ransom BR, Kettenmann H (1993) Development of cell–cell coupling among cells of the oligodendrocyte lineage. Glia 7:322–328
Weiner HL (2009) The Challenge of Multiple Sclerosis: How Do We Cure A Chronic Heterogeneous Disease? Ann Neurol 65:239–248
Wolswijk G (1998) Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci 18(2):601–609
Zamvil SS, Steinman L (2003) Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron 38:685–688
Acknowledgments
This project was funded by the Cyprus Research Promotion Foundation (Grants ACCESS/0308/11 and HEALTH/BIOS/0308/01 to KAK) and by Cyprus Telethon (2010-11 grant to KAK). Post-mortem human brain tissue samples were kindly provided by the UK Multiple Sclerosis Society Tissue Bank, Imperial College London, funded by the UK Multiple Sclerosis Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Markoullis and I. Sargiannidou contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2012_978_MOESM2_ESM.tif
Suppl. Figure 1: Visualization of glial GJ plaques with confocal microscopy. These are images captured with confocal microscopy of non-MS control fixed brain WM sections double immunostained for GJ proteins (green) as indicated and cell or axonal markers (red) including GFAP (b), Iba1 (e), PLP (h), and Kv1.2 (k), as indicated. Merged images are shown in the right column. Diffusely distributed Cx43 GJ plaques are shown in a, while Cx47 GJ plaques are typically concentrated around oligodendrocytes and their proximal processes (green arrowheads in d). Cx32 GJ plaques are diffusely expressed along myelinated fibers (g) but frequently also appear in a paranodal distribution (green arrowheads in j) surrounding juxtaparanodal Kv1.2 channels (red arrowheads in k). Scale bar: 20 μm (TIFF 16053 kb)
401_2012_978_MOESM3_ESM.tif
Suppl. Figure 2: Example of glial GJ plaque counts in control and MS brain sections. Quantification was performed in defined areas of pathologically characterized sections (example schematic diagram in a) immunostained for each of the GJ proteins Cx32, Cx47, and Cx43. GJ plaques were counted in NAWM areas as well as in lesions (L) and periplaque (P1-P3) (example in b; for definitions see Methods) in order to examine the gradient of GJ pathology. GJ plaque counts were performed using the ImagePro Plus software. Representative images with counted GJ plaques (highlighted red) in different areas as indicated are shown for Cx32 (c), for Cx47 (d) and for Cx43 (e). Cell nuclei are stained with DAPI (blue). Scale bars: in b: 100 μm; in c-e: 50 μm (TIFF 16341 kb)
401_2012_978_MOESM4_ESM.tif
Suppl. Figure 3: Gradient of inflammation from MS NAWM toward the lesions. Immunostaining for Iba1 (red) in the same fields in which GJ counts were obtained (control WM, NAWM, perilesion P1-3, and lesions) reveals a gradient of inflammation with increasing immunoreactivity of Iba1-positive (red) activated microglia toward the lesions, reaching a maximum in the perilesion area (a-f). Cell nuclei are stained with DAPI (blue). Scale bar: 50 μm. Quantitative analysis (g) of Iba1 immunoreactivity (average % of total area from multiple fields) from groups of non-MS control and MS samples (n = 6 WM areas from 4 control cases, 5 NAWM areas from 4 MS cases, and 3 lesions and perilesions from 3 MS cases) confirms the presence of significant inflammation in MS NAWM compared to control WM, which increases toward chronic active lesions, reaching a maximum at the immediate border of the lesions (P1) (*indicates significant p-values following Bonferroni-Dunn correction for multiple comparisons) (TIFF 14216 kb)
401_2012_978_MOESM5_ESM.tif
Suppl. Figure 4: Identification and quantification of oligodendrocyte precursor cells. These are images of Images of control WM (a, c) and MS NAWM (b, d) immunostained with NG2 (a, b, green) or Olig2 (c, d, red) antibodies to identify oligodendrocyte precursor cells (OPCs). Cell nuclei are stained with DAPI (blue). Staining with both NG2 and Olig2 shows higher OPC numbers (white arrowheads) in MS compared to control WM. Scale bar: 20 μm. OPC counts (e) obtained in different areas of control WM (n = 9 areas from 3 cases) and MS NAWM (n = 9 areas from 3 cases) demonstrate that OPCs are significantly increased in MS brain (MS) compared to non-MS controls (C). Scale bar: 30 μm (TIFF 10344 kb)
401_2012_978_MOESM6_ESM.tif
Suppl. Figure 5: Lack of Cx32 expression in oligodendrocyte precursor cells. Images of double immunostaining for Cx32 (a, d, green) and Olig2 (b, e, red) in control WM (a-c) and in MS NAWM (d-f). Cell nuclei are stained with DAPI and merged images are shown in the right collumn (c, f). Although many more OPCs are present in MS tissue (red arrows) compared to control WM, they do not colocalize with Cx32 immunoreactivity (green arrowheads), which is found mainly along myelinated fibers both in control WM and in MS NAWM. Scale bars: 10 μm (TIFF 6157 kb)
401_2012_978_MOESM7_ESM.tif
Suppl. Figure 6: Increased Cx43 levels in MS lesions: Immunoblot analysis for Cx43 (a) in control WM (n = 2 cases), MS NAWM (n = 2 cases) and in 5 different lesions (L) (n = 5 cases) shows overall increased levels in MS, although with some variability. GAPDH blot was used for loading control. Quantitative analysis (b) shows significantly elevated Cx43 levels in MS lesions compared to control WM (*indicates significant p-values following Bonferroni-Dunn correction for multiple comparisons) (TIFF 3476 kb)
401_2012_978_MOESM8_ESM.tif
Suppl. Figure 7: Expression of Cx30 in the cortex of control and MS brain. These are merged (a-f) or separate and merged channels (g-l) images from immunostaining for Cx30 (red) and Cx43 (green) in MS and non-MS brain, as indicated. Cell nuclei are stained with DAPI (blue). At low magnification (a, d) it is apparent that Cx30 is almost exclusively expressed in the cortex (a), where it forms dense GJ plaques shown at higher magnification (b). Cx30 is not expressed in the WM, and not in or around MS lesions (d). This is in contrast to Cx43, which is most prominent in the WM (f) and NAWM (a), and in MS lesions (d). Higher magnification images of MS NAWM (c) and MS lesion (e) show prominent Cx43 expression but no Cx30 expression. Lack of Cx30 expression is also found in control WM (f), and is therefore not a feature of MS. Higher magnification separate channels of MS cortex (g-i) and NAWM (j-l) show the characteristic diffuse GJ plaque formation by Cx30 in the cortex, typically decorating the surface of neurons (red arrowheads in g), and lack of Cx30 GJs in the NAWM (j). Cx43 (green arrowheads) is more distinctly expressed forming GJ plaques around astrocytes and blood vessels both in the cortex (h) and NAWM (k). Scale bars in a and d: 100 μm; in b, c, e: 20 μm; in f and g-l as well as in insets of b and e: 10 μm (TIFF 14473 kb)
401_2012_978_MOESM9_ESM.tif
Suppl. Figure 8: Decreasing colocalization of Cx47 and Cx43 around lesions and in the NAWM. These are images (merged channels in a-d and both separate and merged channels at higher magnification in e & f) of double labelling for Cx47 (red) and Cx43 (green) in control WM (a, e) and in MS NAWM (b), perilesion (c, f), or lesion (d). Cell nuclei are stained with DAPI (blue). GJ plaques formed by Cx47 and Cx43 colocalize around oligodendrocytes in control WM (white arrowheads in a and e). This colocalization is decreased in MS NAWM (b) and perilesion (c, f), where smaller Cx47 GJ plaques (red arrowheads) can be seen that do not overlap with Cx43 immunoreactivity. This is despite increasing density of Cx43 GJ plaques (green arrowheads) that are frequently concentrated around activated astrocytes, most prominently in the lesion (white arrows in d), where Cx47 is almost absent. Scale bars: in a-d: 50 μm; in e–f: 10 μm (TIFF 5661 kb)
Rights and permissions
About this article
Cite this article
Markoullis, K., Sargiannidou, I., Schiza, N. et al. Gap junction pathology in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol 123, 873–886 (2012). https://doi.org/10.1007/s00401-012-0978-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-0978-4