Abstract
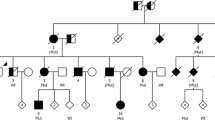
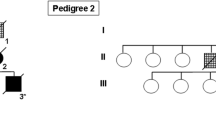
In order to determine the frequency of microtubule-associated protein tau gene (MAPT) mutations and rare variants in CBD, we performed a systematic sequence analysis of MAPT coding and 3′ untranslated region (3′UTR) in a large cohort of autopsy-confirmed CBD patients (N = 109). This identified a novel MAPT mutation in exon 13, p.N410H, in a case that is neuropathologically indistinguishable from sporadic CBD. On immunoblot, the p.N410H mutation carrier had the same insoluble tau profile as seen in CBD. Additionally, tau expression analysis in brain tissue found a significant increase in the 4R/3R tau mRNA ratio (P = 0.04), indicating that p.N410H disrupts tau isoform homeostasis. Biochemically, recombinant tau protein with p.N410H showed a marked increase in tau filament formation compared to wild-type tau (P < 0.001), had a 19.2 % decrease in rate of microtubule assembly (P < 0.05), and a 10.3 % reduction in the extent of total microtubule polymerization (P < 0.01). Sequence analysis of the complete MAPT 3′UTR in autopsy-confirmed CBD cases further identified two rare variants with nominally significant association with CBD. An ATC nucleotide insertion (“MAPTv8”) was found in 4.6 % of CBD patients compared to 1.2 % of controls (P = 0.031, OR = 3.71), and rs186977284 in 4.6 % CBD patients, but only 0.9 % of controls (P = 0.04, OR = 3.58). Rs186977284 was also present in 2.7 % of a large cohort of autopsy-confirmed PSP patients (N = 566) and only 0.9 % of an additional control series (P = 0.034, OR = 3.08), extending the association to PSP. Our findings show that mutations in MAPT can cause CBD and MAPT non-coding variants may increase the risk of complex 4R tauopathies.





Similar content being viewed by others
References
Adams SJ, DeTure MA, McBride M, Dickson DW, Petrucelli L (2010) Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS ONE 5:e10810
Ahmed Z, Josephs KA, Gonzalez J, DelleDonne A, Dickson DW (2008) Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain 131:460–472
Baker M, Litvan I, Houlden H et al (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8:711–715
Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC (1999) Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 53:795–800
Bugiani O, Murrell JR, Giaccone G et al (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 58:667–677
Conrad C, Andreadis A, Trojanowski JQ et al (1997) Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol 41:277–281
Coppola G, Chinnathambi S, Lee JJ et al (2012) Evidence for a role of the rare p. A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet 21:3500–3512
Di Noto L, DeTure MA, Purich DL (1999) Disulfide-cross-linked tau and MAP2 homodimers readily promote microtubule assembly. Mol Cell Biol Res Commun 2:71–76
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(Suppl 2):II6–II15
Dickson DW, Bergeron C, Chin SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Ezquerra M, Pastor P, Valldeoriola F, Molinuevo JL, Blesa R, Tolosa E, Oliva R (1999) Identification of a novel polymorphism in the promoter region of the tau gene highly associated to progressive supranuclear palsy in humans. Neurosci Lett 275:183–186
Fekete R, Bainbridge M, Baizabal-Carvallo JF, Rivera A, Miller B, Du P, Kholodovych V, Powell S, Ondo W (2013) Exome sequencing in familial corticobasal degeneration. Parkinsonism Relat Disord. pii: S1353-8020(13)00234-4
Geser F, Winton MJ, Kwong LK et al (2008) Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115:133–145
Grover A, England E, Baker M et al (2003) A novel tau mutation in exon 9 (1260V) causes a four-repeat tauopathy. Exp Neurol 184:131–140
Hoglinger GU, Melhem NM, Dickson DW et al (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43:699–705
Houlden H, Baker M, Morris HR et al (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56:1702–1706
Hutton M, Lendon CL, Rizzu P et al (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705
Ingelsson M, Ramasamy K, Cantuti-Castelvetri I et al (2006) No alteration in tau exon 10 alternative splicing in tangle-bearing neurons of the Alzheimer’s disease brain. Acta Neuropathol 112:439–449
Kara E, Ling H, Pittman AM et al (2012) The MAPT p.A152T variant is a risk factor associated with tauopathies with atypical clinical and neuropathological features. Neurobiol Aging 33:2231, e2237–e2231, e2214
Kouri N, Murray ME, Hassan A et al (2011) Neuropathological features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain 134:3264–3275
Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE, Yen SH, Dickson DW (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752
Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW (2011) Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 7:263–272
Kovacs GG, Wohrer A, Strobel T, Botond G, Attems J, Budka H (2011) Unclassifiable tauopathy associated with an A152T variation in MAPT exon 7. Clin Neuropathol 30:3–10
Lippa CF, Zhukareva V, Kawarai T et al (2000) Frontotemporal dementia with novel tau pathology and a Glu342Val tau mutation. Ann Neurol 48:850–858
McKee AC, Gavett BE, Stern RA et al (2010) TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69:918–929
Nasreddine ZS, Loginov M, Clark LN et al (1999) From genotype to phenotype: a clinical pathological, and biochemical investigation of frontotemporal dementia and parkinsonism (FTDP-17) caused by the P301L tau mutation. Ann Neurol 45:704–715
Pastor P, Ezquerra M, Perez JC et al (2004) Novel haplotypes in 17q21 are associated with progressive supranuclear palsy. Ann Neurol 56:249–258
Poorkaj P, Bird TD, Wijsman E et al (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Rademakers R, Melquist S, Cruts M et al (2005) High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet 14:3281–3292
Santacruz K, Lewis J, Spires T et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA 95:7737–7741
Spillantini MG, Yoshida H, Rizzini C, Lantos PL, Khan N, Rossor MN, Goedert M, Brown J (2000) A novel tau mutation (N296N) in familial dementia with swollen achromatic neurons and corticobasal inclusion bodies. Ann Neurol 48:939–943
Uryu K, Nakashima-Yasuda H, Forman MS et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
Williams DR, Holton JL, Strand K, Revesz T, Lees AJ (2007) Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord 22:2235–2241
Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML (2003) Hereditary tauopathies and parkinsonism. Adv Neurol 91:153–163
Zarranz JJ, Ferrer I, Lezcano E et al (2005) A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64:1578–1585
Acknowledgments
The authors would like to thank the patients and their families for support of this research. We would also like to thank Mariely De Jesus-Hernandez for sequencing assistance, Monica Castanedes Casey, Linda Rousseau, and Virginia Phillips for histological and immunohistochemistry assistance, and Beth Marten for logistic and administrative support in collecting material for this study. This work was funded by the Irene and Abe Pollin Fund for CBD. DWD is supported by the Mayo Foundation (Jacoby Professorship of Alzheimer Research) and NIH grants: P50-AG016574, P50-NS072187 and P01-AG003949. The Society for Progressive Supranuclear Palsy Brain Bank is supported by CurePSP. OAR is supported by NINDS NS078086 and P50 NS072187. ZKW is partially supported by the NIH/NINDS P50 NS072187, Mayo Clinic Center for Regenerative Medicine, Dystonia Medical Research Foundation, The Michael J. Fox Foundation for Parkinson’s Research, and the gift from Carl Edward Bolch, Jr. and Susan Bass Bolch. KAJ is supported by the NIH grants R01-AG037491 and R01-DC010367.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kouri, N., Carlomagno, Y., Baker, M. et al. Novel mutation in MAPT exon 13 (p.N410H) causes corticobasal degeneration. Acta Neuropathol 127, 271–282 (2014). https://doi.org/10.1007/s00401-013-1193-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1193-7