Abstract
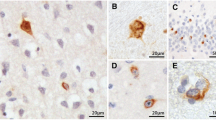
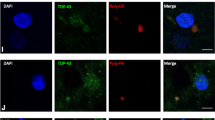
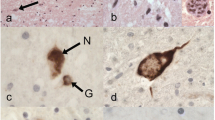
We examined the phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) inclusions as well as neuronal loss in full-length spinal cords and five selected regions of the central nervous system from 36 patients with amyotrophic lateral sclerosis (ALS) and 10 age-matched normal controls. The most severe neuronal loss and pTDP-43 lesions were seen in lamina IX motor nuclei columns 4, 6, and 8 of lower cervical segments and in columns 9–11 of lumbosacral segments. Severity of pTDP-43 pathology and neuronal loss correlated closely with gray and white matter oligodendroglial involvement and was linked to onset of disease, with severe involvement of columns 4, 6, and 8 of upper extremity onset cases and severe involvement of columns of 9, 10, and 11 in cases with lower extremity onset. Severe TDP-43 lesions and neuronal loss were observed in stage 4 cases and sometimes included Onuf’s nucleus. Notably, three cases displayed pTDP-43 aggregates in the midbrain oculomotor nucleus, which we had not seen previously even in cases with advanced (i.e., stage 4) pathology. pTDP-43 aggregates were observed in neurons of Clarke’s column in 30.6 % of cases but rarely in the intermediolateral nucleus (IML). Gray matter oligodendroglial pTDP-43 inclusions were present in areas devoid of neuronal pTDP-43 aggregates and neuronal loss. Taken together, our findings indicate that (1) the dorsolateral motor nuclei columns of the cervical and lumbosacral anterior horn may be the earliest foci of pTDP-43 pathology in the spinal cord, (2) gray matter oligodendroglial involvement is an early event in the ALS disease process that possibly heralds subsequent involvement of neurons by pTDP-43 pathology, and (3) in some very advanced cases, there is oculomotor nucleus involvement, which may constitute an additional neuropathological stage (designated here as stage 5) of pTDP-43 pathology in ALS.





Similar content being viewed by others
References
Braak H, Alafuzoff I, Arzberger T et al (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Brettschneider J, Ludolph AC et al (2013) Amyotrophic lateral sclerosis-a model of corticofugal axonal spread. Nat Rev Neurol 9:708–714
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121:171–181
Braak H, Del Tredici K (2012) Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol 25:708–714
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Ludolph A, Thal DR et al (2010) Amyotrophic lateral sclerosis: dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol 120:67–74
Brettschneider J, Del Tredici K, Irwin DJ et al (2014) Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 127:423–439
Brettschneider J, Del Tredici K, Toledo JB et al (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38
Brettschneider J, Libon DJ, Toledo JB et al (2012) Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol 123:395–407
Brettschneider J, Toledo JB, Van Deerlin VM et al (2012) Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS One 7:e39216
Brettschneider J, Van Deerlin VM, Robinson JL et al (2012) Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol 123:825–839
Brooks BR, Miller RG, Swash M et al (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Brown LT (1974) Rubrospinal projections in the rat. J Comp Neurol 154:169–187
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Cooper-Knock J, Hewitt C, Highley JR et al (2012) Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 135:751–764
Donaghy C, Thurtell MJ, Pioro EP et al (2011) Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry 82:110–116
Duda JE, Giasson BI, Mabon ME et al (2002) Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 52:205–210
Fallini C, Bassell GJ, Rossoll W (2012) The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Hum Mol Genet 21:3703–3718
Feldengut S, Del Tredici K, Braak H (2013) Paraffin sections of 70–100 mum: a novel technique and its benefits for studying the nervous system. J Neurosci Methods 215:241–244
Geser F, Brandmeir NJ, Kwong LK et al (2008) Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 65:636–641
Geser F, Martinez-Lage M, Robinson J et al (2009) Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 66:180–189
Guo JL, Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20:130–138
Harvey DG, Torack RM, Rosenbaum HE (1979) Amyotrophic lateral sclerosis with ophthalmoplegia. A clinicopathologic study. Arch Neurol 36:615–617
Horner PJ, Thallmair M, Gage FH (2002) Defining the NG2-expressing cell of the adult CNS. J Neurocytol 31:469–480
Iwanaga K, Hayashi S, Oyake M et al (1997) Neuropathology of sporadic amyotrophic lateral sclerosis of long duration. J Neurol Sci 146:139–143
Jenny AB, Inukai J (1983) Principles of motor organization of the monkey cervical spinal cord. J Neurosci 3:567–575
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51
Kang SH, Li Y, Fukaya M et al (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16:571–579
Keller BA, Volkening K, Droppelmann CA et al (2012) Co-aggregation of RNA binding proteins in ALS spinal motor neurons: evidence of a common pathogenic mechanism. Acta Neuropathol 124:733–747
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955
Kimura T, Jiang H, Konno T et al. (2014) Bunina bodies in motor and non-motor neurons revisited: a pathological study of an ALS patient after long-term survival on a respirator. Neuropathology
Komachi H, Okeda R, Ishii N et al (1994) Motor neuron disease with dementia and ophthalmoplegia. A clinical and pathological study. J Neurol 241:592–596
Lee EB, Lee VM, Trojanowski JQ (2012) Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci 13:38–50
Lee EB, Leng LZ, Zhang B et al (2006) Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem 281:4292–4299
Lee Y, Morrison BM, Li Y et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448
Ling SC, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79:416–438
Miki Y, Mori F, Nunomura J et al (2010) Sporadic amyotrophic lateral sclerosis with pallido-nigro-luysian degeneration: a TDP-43 immunohistochemical study. Neuropathology 30:149–153
Mizuno Y, Fujita Y, Takatama M et al (2012) Comparison of phosphorylated TDP-43-positive inclusions in oculomotor neurons in patients with non-ALS and ALS disorders. J Neurol Sci 315:20–25
Mori F, Tanji K, Zhang HX et al (2008) Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116:193–203
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishihira Y, Tan CF, Hoshi Y et al (2009) Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol 117:45–53
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Nonaka T, Masuda-Suzukake M, Arai T et al (2013) Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 4:124–134
Phukan J, Pender NP, Hardiman O (2007) Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 6:994–1003
Ravits J, Paul P, Jorg C (2007) Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 68:1571–1575
Ravits JM, La Spada AR (2009) ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology 73:805–811
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Riku Y, Watanabe H, Yoshida M et al (2014) Lower motor neuron involvement in TAR DNA-binding protein of 43 kDa-related frontotemporal lobar degeneration and amyotrophic lateral sclerosis. JAMA Neurol 71:172–179
Routal RV, Pal GP (1999) A study of motoneuron groups and motor columns of the human spinal cord. J Anat 195(Pt 2):211–224
Rowland LP (2001) How amyotrophic lateral sclerosis got its name: the clinical-pathologic genius of Jean-Martin Charcot. Arch Neurol 58:512–515
Schoenen J (1982) Dendritic organization of the human spinal cord: the motoneurons. J Comp Neurol 211:226–247
Sharrard WJ (1955) The distribution of the permanent paralysis in the lower limb in poliomyelitis: a clinical and pathological study. J Bone Jt Surg Br 37-B:540–558
Stewart H, Rutherford NJ, Briemberg H et al (2012) Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathol 123:409–417
Sumi H, Kato S, Mochimaru Y et al (2009) Nuclear TAR DNA binding protein 43 expression in spinal cord neurons correlates with the clinical course in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 68:37–47
Takeda T, Uchihara T, Nakayama Y et al (2014) Dendritic retraction, but not atrophy, is consistent in amyotrophic lateral sclerosis-comparison between Onuf’s neurons and other sacral motor neurons. Acta Neuropathol Commun 2:11
Terman JR, Wang XM, Martin GF (1998) Origin, course, and laterality of spinocerebellar axons in the North American opossum, Didelphis virginiana. Anat Rec 251:528–547
Thal DR, Rub U, Orantes M et al (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Toledo JB, Van Deerlin VM, Lee EB et al (2013) A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement
Van Rheenen W, Van Blitterswijk M, Huisman MH et al (2012) Hexanucleotide repeat expansions in C9ORF72 in the spectrum of motor neuron diseases. Neurology 79:878–882
Waldron HA, Gwyn DG (1969) Descending nerve tracts in the spinal cord of the rat. I. Fibers from the midbrain. J Comp Neurol 137:143–153
Xie SX, Baek Y, Grossman M et al (2011) Building an integrated neurodegenerative disease database at an academic health center. Alzheimers Dement 7:e84–e93
Zhang H, Tan CF, Mori F et al (2008) TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 115:115–122
Acknowledgments
We thank the patients who contributed to this study. We are also grateful to Kevin Raible, Terry Schuck, Sigrid Baumann, Gabriele Ehmke, Simone Feldengut, Julia Straub, and Thi Phuong Thu Brettschneider for technical support, together with David Ewert (University of Ulm) for assistance with the graphics. This study was supported by the NIH (AG033101, AG017586, AG010124, AG032953, AG039510, NS044266), the Wyncote Foundation, and the Koller Family Foundation, as well as by the German Motor Neuron Disease Network. VM-YL is the John H. Ware, 3rd, Professor of Alzheimer’s Disease Research. JQT is the William Maul Measey-Truman G. Schnabel, Jr. Professor of Geriatric Medicine and Gerontology. DJI is supported by the Penn Institute for Translational Medicine & Therapeutics (ITMAT). HB and KDT are supported by the Michael J. Fox Foundation for Parkinson’s Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Brettschneider, K. Arai, and K. Del Tredici contributed equally.
H. Braak and J. Q. Trojanowski are Senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brettschneider, J., Arai, K., Del Tredici, K. et al. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol 128, 423–437 (2014). https://doi.org/10.1007/s00401-014-1299-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1299-6