Abstract
Objective
To delineate the MRI features that distinguish neuromyelitis optica (NMO) from multiple sclerosis (MS).
Methods
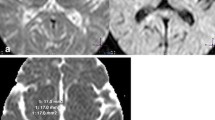
We compared the distribution of the spinal cord lesions by analyzing 1) lesion area, 2) lesion density (by superimposing the lesions onto the standard sections of the cervical and thoracic cord with appropriate transparencies using computer software), and 3) T1-hypointensity in axial sections of MRI in NMO and MS.
Results
In NMO, 60–70% of the cervical and thoracic cord MRI lesions occupied more than half of the cord area and mainly involved the central gray matter in the acute stage. In the chronic stage, half or more of the lesions were localized at the central gray matter region. The lesion superimposition analysis also revealed much higher densities in the central gray matter region than in the peripheral white matter regions. Two patients with NMO had T1-hypointense lesions in the central region. In contrast, over 80% of the lesions in MS were localized in the lateral and posterior white matter regions of the cord in the chronic as well as acute stage. Lesion densities were much higher in the lateral and posterior white matter regions than in the central gray matter region. None of the lesions in MS were T1-hypointense.
Conclusions
These MRI findings strongly suggest a preferential involvement in the spinal central gray matter in NMO which is distinct from MS.
Similar content being viewed by others
References
Benedetti B, Valsasina P, Judica E, Martinelli V, Ghezzi A, Capra R, Bergamaschi R, Comi G, Filippi M (2006) Grading cervical cord damage in neuromyelitis optica and MS by diffusion tensor MRI. Neurology 67:161–163
Cordonnier C, de Seze J, Breteau G, Ferriby D, Michelin E, Stojkovic T, Pruvo JP, Vermersch P (2003) Prospective study of patients presenting with acute partial transverse myelopathy. J Neurol 250:1447–1452
de Seze J, Stojkovic T, Ferriby D, Gauvrit JY, Montagne C, Mounier- Vehier F, Verier A, Pruvo JP, Hache JC, Vermersch P (2002) Devic's neuromyelitis optica: clinical, laboratory, MRI and outcome profile. J Neurol Sci 197:57–61
Filippi M, Rocca MA (2004) MR imaging of Devic's neuromyelitis optica. Neurol Sci 25(Suppl 4):S371–S373
Filippi M, Rocca MA, Moiola L, Martinelli V, Ghezzi A, Capra R, Salvi F, Comi G (1999) MRI and magnetization transfer imaging changes in the brain and cervical cord of patients with Devic's neuromyelitis optica. Neurology 53:1705–1710
Frydenlund DS, Bhardwaj A, Otsuka T, Mylonakou MN, Yasumura T, Davidson KG, Zeynalov E, Skare O, Laake P, Haug FM, Rash JE, Agre P, Ottersen OP, Amiry-Moghaddam M (2006) Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci USA 103:13532–13536
Ghezzi A, Bergamaschi R, Martinelli V, Trojano M, Tola MR, Merelli E, Mancardi L, Gallo P, Filippi M, Zaffaroni M, Comi G; Italian Devic's Study Group (IDESG) (2004) Clinical characteristics, course and prognosis of relapsing Devic's Neuromyelitis Optica. J Neurol 251:47–52
Gilmore CP, Bö L, Owens T, Lowe J, Esiri MM, Evangelou N (2006) Spinal cord gray matter demyelination in multiple sclerosis – a novel pattern of residual plaque morphology. Brain Pathol 16:202–208
Haines DE (1995) Neuroanatomy: An Atlas of Structures, Sections, and Systems. Fourth edition. Philadelphia: Lippincott Williams and Wilkins
Ishida H, Takemori K, Dote K, Ito H (2006) Expression of glucose transporter- 1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood-brain barrier function. Am J Hypertens 19:33–39
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202:473–477
Losseff NA, Wang L, Miller DH, Thompson AJ (2001) T1 hypointensity of the spinal cord in multiple sclerosis. J Neurol 248:517–521
Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H (2002) A role for humoral mechanism in the pathogenesis of Devic's neuromyelitis optica. Brain 125:1450–1461
Mandler RN, Davis LE, Jeffery DR, Kornfeld M (1993) Devic's neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol 34:162–168
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Misu T, Fujihara K, Nakashima I, Miyazawa I, Okita N, Takase S, Itoyama Y (2002) Pure optic-spinal form of multiple sclerosis in Japan. Brain 125:2460–2468
Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y (2005) Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 65:1479–1482
Misu T, Kakita A, Fujihara K, Nakashima I, Takahashi H, Itoyama Y (2005) A comparative neuropathological analysis of Japanese cases of neuromyelitis optica and multiple sclerosis. Neurology 64(Suppl 1):A39
Misu T, Fujihara K, Nakamura M, Murakami K, Endo M, Konno H, Itoyama Y (2006) Loss of aquaporin-4 in active perivascular lesions in neuromyelitis optica: a case report. Tohoku J Exp Med 209:269–275
Nakamura M, Endo M, Murakami K, Konno H, Fujihara K, Itoyama Y (2005) An autopsied case of neuromyelitis optica with large cavitary cerebral lesion. Mult Scler 11:735–738
Nakashima I, Fujihara K, Okita N, Takase S, Itoyama Y (1999) Clinical and MRI study of brain stem and cerebellar involvement in Japanese patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 67:153–157
Nakashima I, Fujihara K, Sato S, Itoyama Y (2005) Oligoclonal IgG bands in Japanese patients with multiple sclerosis. A comparative study between isoelectric focusing with IgG immunofixation and high-resolution agarose gel electrophoresis. J Neuroimmunol 159:133–136
O'Riordan JI, Gallagher HL, Thompson AJ, Howard RS, Kingsley DP, Thompson EJ, McDonaldWI, Miller DH (1996) Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry 60:382–387
Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA (2006) Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 63:964–968
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 58:840–846
Poppe AY, Lapierre Y, Melancon D, Lowden D, Wardell L, Fullerton LM, Bar-Or A (2005) Neuromyelitis optica with hypothalamic involvement. Mult Scler 11:617–621
Rocca MA, Agosta F, Mezzapesa DM, Martinelli V, Salvi F, Ghezzi A, Bergamaschi R, Comi G, Filippi M (2004) Magnetization transfer and diffusion tensor MRI show gray matter damage in neuromyelitis optica. Neurology 62:476–478
Simnad VI, Pisani DE, Rose JW (1997) Multiple sclerosis presenting as transverse myelopathy: Clinical and MRI features. Neurology 48:65–73
Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M (1995) Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 195:725–732
Vernant JC, Cabre P, Smadja D, Merle H, Caubarrere I, Mikol J, Poser CM (1997) Recurrent optic neuromyelitis with endocrinopathies: a new syndrome. Neurology 48:58–64
Watanabe S, Nakashima I, Miyazawa I, Misu T, Shiga Y, Nakagawa Y, Fujihara K, Itoyama Y (in press) Successful treatment of a hypothalamic lesion in neuromyelitis optica by plasma exchange. J Neurol (in press)
Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG (1999) The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 53:1107–1114
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG (2006) Revised diagnostic criteria for neuromyelitis optica. Neurology 66:1485–1489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Miyazawa, I., Fujihara, K. et al. Preferential spinal central gray matter involvement in neuromyelitis optica. J Neurol 255, 163–170 (2008). https://doi.org/10.1007/s00415-008-0545-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0545-z