Abstract
The clinical and neuroanatomical correlates of specific apraxias in neurodegenerative disease are not well understood. Here we addressed this issue in progressive nonfluent aphasia (PNFA), a canonical subtype of frontotemporal lobar degeneration that has been consistently associated with apraxia of speech (AOS) and in some cases orofacial apraxia, limb apraxia and/or parkinsonism. Sixteen patients with PNFA according to current consensus criteria were studied. Three patients had a corticobasal syndrome (CBS) and two a progressive supranuclear palsy (PSP) syndrome. Speech, orofacial and limb praxis functions were assessed using the Apraxia Battery for Adults-2 and a voxel-based morphometry (VBM) analysis was conducted on brain MRI scans from the patient cohort in order to identify neuroanatomical correlates. All patients had AOS based on reduced diadochokinetic rate, 69% of cases had an abnormal orofacial apraxia score and 44% of cases (including the three CBS cases and one case with PSP) had an abnormal limb apraxia score. Severity of orofacial apraxia (but not AOS or limb apraxia) correlated with estimated clinical disease duration. The VBM analysis identified distinct neuroanatomical bases for each form of apraxia: the severity of AOS correlated with left posterior inferior frontal lobe atrophy; orofacial apraxia with left middle frontal, premotor and supplementary motor cortical atrophy; and limb apraxia with left inferior parietal lobe atrophy. Our findings show that apraxia of various kinds can be a clinical issue in PNFA and demonstrate that specific apraxias are clinically and anatomically dissociable within this population of patients.
Similar content being viewed by others
Introduction
Apraxia can be defined as a higher order motor disorder of skilled and/or learned movements [1]. The motor control deficit in apraxia may be specific for particular movements or body parts: amongst these, apraxia of limb movements is most often described, however apraxias of the cranial musculature (orofacial apraxia: [2]) and apraxia of the finely coordinated movements of articulation (apraxia of speech, AOS: [3]) are also well recognised. The nature and brain basis for these specific disorders of voluntary action have not been fully defined, and apraxia remains an issue of considerable neurobiological as well as clinical interest. Anatomical evidence, chiefly from patients with stroke, has implicated distributed cerebral circuitry in the voluntary control of orofacial and limb movements and the production of apraxias [4, 5]: for orofacial apraxia, prefrontal areas and their subcortical projections are particularly implicated whilst for limb apraxia more posterior areas, particularly the parietal lobe and its connections appear to be most commonly involved [4, 5]. Aphasia (and in particular, impaired speech production) has often been documented in association with apraxia [5], and frontoparietal circuits in the dominant hemisphere have also been implicated in the programming of speech sounds and in association with AOS, with particular emphasis on the insula and posterior left inferior frontal gyrus (‘Broca’s area’) [6–9]. However, the relations between these different forms of apraxia and their precise anatomical substrates remain contentious.
Progressive nonfluent aphasia (PNFA) is a neurodegenerative disorder considered to be one of the primary progressive aphasias (PPA) and falling within the frontotemporal lobar degeneration (FTLD) spectrum [10, 11]. Although the term PNFA was originally considered to include all patients with progressively “nonfluent” speech of any cause [5], some recent studies have limited PNFA to include only those patients with motor speech impairment and/or expressive agrammatism [12, 13]. In particular, these studies have stressed the importance of apraxia of speech (AOS) as a defining feature of this group of patients [14]: AOS is a motor speech disorder with the features of hesitancy, effortfulness with articulatory groping, phonetic errors and dysprosody [3, 15]. PNFA may be associated clinically with parkinsonian syndromes, in particular either a corticobasal degeneration syndrome (CBS) or a progressive supranuclear palsy (PSP) syndrome. At post mortem, abnormal tau inclusions are often seen in PNFA, with the 4-repeat tauopathies of corticobasal degeneration or PSP common underlying pathologies [16, 17]. Limb apraxia is a well-known feature of CBS [18] and can also occur with PSP syndromes [19, 20]. Although less well studied, orofacial apraxia may also develop in CBS [21, 22]. The clinico-pathological overlap of CBS and PSP with PNFA, coupled with the central role of AOS in the PNFA syndrome, suggests that apraxia of different kinds may be clinically relevant in PNFA. Both orofacial (or buccofacial) apraxia [23–27] and limb apraxia [28] have been reported in PNFA, however these associations have not been studied systematically. Furthermore, although AOS has been associated with atrophy in the left frontal lobe and insula [14, 16], the neuroanatomical correlates of the apraxias accompanying focal dementia syndromes have not been established. In this study, we assessed speech, orofacial and limb praxis in a cohort of patients with PNFA and assessed neuroanatomical correlates of the corresponding apraxis using the semi-automated and unbiased technique of voxel-based morphometry (VBM).
Methods
Sixteen patients with a diagnosis of PNFA according to current consensus criteria [11, 12] were recruited from the tertiary Specialist Cognitive Disorders Clinic of the National Hospital of Neurology and Neurosurgery, London, UK. The PNFA cohort comprised 12 men and 4 women with a mean (standard deviation) age at assessment of 72.1 (+/−6.9) years and disease duration from symptom onset of 5.8 (+/−2.1) years. Five patients had parkinsonian features when assessed: three had CBS, and two PSP. Research ethics approval for this study was obtained from the National Hospital for Neurology and Neurosurgery and University College London Hospitals Research Ethics Committees.
Apraxia analysis
We used subscores from the Apraxia Battery for Adults-2 (ABA-2 [29]) as measures of apraxia. Diadochokinetic (DDK) rate score (ABA-2 subtest 1) was measured by asking patients to repeat the phrases “puh-tuh”, “tuh-kuh”, “puh-tuh-kuh” and “pluh-kruh-tuh” as many times as possible in 3 s (for two syllable phrases) and 5 s (for three syllable phrases) for a maximum of three trials, and the sum of the best trials from each of the four phrases was used as the total score. DDK rate for alternating syllables is particularly sensitive to the presence of AOS [30] and here is used as a surrogate measure of AOS severity. Orofacial apraxia score was based on ABA-2 subtest 3B in which patients were asked to perform the following actions: stick out your tongue, whistle, puff out your cheeks, pretend to kiss, clear your throat, bite your lower lip, show me your teeth, take a deep breath and hold it, lick your lips and open your mouth. Each action was scored out of 5 (i.e., maximum score was 50): a score of 5 was assigned when the subject made an accurate, prompt, complete and readable gesture; 4 when the subject made an ambiguous or incorrect gesture, but self corrected to an accurate response, 3 when the subject’s gesture was essentially correct, but crude and defective in amplitude, speed or accuracy. If the subject made no response after ten seconds, or attempted a response but was unsuccessful, the gesture was demonstrated by the examiner and scores were assigned as follows: 2 when the subject performed correctly after demonstration, 1 when the subject’s gesture, after demonstration, was essentially correct, but crude and defective in amplitude, speed or accuracy, and 0 when, even after demonstration, the subject was unable to perform the correct gesture. Limb apraxia score was based on ABA-2 subtest 3A in which patients were asked to perform the following gestures: make a fist, wave goodbye, snap your fingers, throw a ball, hide your eyes, make a hitch-hiking sign, make a pointing sign, salute, play the piano and scratch. Scoring was as for orofacial praxis with a maximum score of 50.
VBM analysis
MR brain images were acquired on a 1.5T GE Signa scanner (General Electric, Milwaukee, WI). T1-weighted volumetric images were obtained with a 24-cm field of view and 256 × 256 matrix to provide 124 contiguous 1.5-mm-thick slices in the coronal plane (TE = 5 ms, TR = 12 ms, TI = 650 ms). VBM was implemented using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm) with default settings for all parameters. Brain images underwent an initial segmentation process in SPM5 which simultaneously estimated transformation parameters for warping grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue probability maps (TPMs) onto the images. The native space GM segments were then rigidly spatially normalised, using just the rotations and translations from the inverse of the TPM transformation, and resampled to 1.5 mm isotropic resolution. These “imported” images were then iteratively warped to an evolving estimate of their group-wise GM average template using the DARTEL toolbox [31, 32]. The GM segmentations were then normalised using the final DARTEL transformations and modulated to account for volume changes. Finally, the images were smoothed using a 6-mm full-width at half-maximum (FWHM) Gaussian kernel. Linear regression models were used to examine changes in GM volume as functions of apraxia of speech (as measured by diadochokinetic rate score, ABA-2 subtest 1), orofacial apraxia (ABA-2 subtest 3B score) and limb apraxia (ABA-2 subtest 3A score) across the PPA group. Voxel intensity, V, was modelled as a function of praxis score, combining scores for AOS and orofacial apraxia and separately for each apraxia subtype, with subject age and total intracranial volume (TIV) included as nuisance covariates. V = β 1 apraxia + β 2 age + β 3 TIV + μ + ε (where μ is a constant, and ε is error). The analysis was performed over voxels inside a ‘consensus mask’ [33], which included all voxels where intensity >0.1 was present in >70% of subjects. Maps showing statistically significant correlations were generated, uncorrected at a 0.001 significance threshold. Statistical parametric maps were displayed as overlays on a study-specific template, created by warping all native space whole-brain images to the final DARTEL template and calculating the average of the warped brain images.
Results
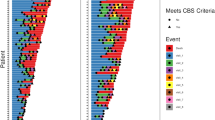
All patients scored in the abnormal range for DDK rate (AOS): most (69%) scored in the mildly impaired range, 19% in the moderate range and 13% in the severe range (Fig. 1a). For the orofacial apraxia measure, 69% of patients (11 of 16) scored within the abnormal range (50% mild, 13% moderate and 6% severe) (Fig. 1b). This included all of the patients with either CBS or a PSP syndrome. Patients without orofacial apraxia tended to be those scoring in the mildly impaired range for DDK rate (4 of 5) with only one patient scoring normally but in the moderately impaired range for DDK rate. A substantial minority of PNFA patients had limb apraxia, 44% (7 patients) scoring in the abnormal range (Fig. 1c). These seven cases included the three patients with CBS and one of the patients with PSP; i.e. three patients with limb apraxia did not have a CBS or PSP syndrome. For the orofacial apraxia score there was a correlation with estimated clinical disease duration (p = 0.04); no such correlation was found for DDK rate score (p = 0.23) or limb praxis (p = 0.38). Although we did not assess patients formally for the presence of swallowing apraxia, it is noteworthy that none of the patients included in this study reported clinical dysphagia.
In the VBM analysis, the combined praxis score for AOS and orofacial apraxia correlated with grey matter in a left premotor, dorsolateral and inferior frontal cortical network. Distinct correlates of different forms of apraxia were identified when scores were modelled separately. Reduced DDK rate (AOS) correlated with grey matter loss in the left posterior inferior frontal gyrus (pars opercularis of Broca’s area, Brodmann area 45) (Fig. 2a). Orofacial apraxia correlated with grey matter loss in the left middle frontal gyrus (Brodmann area 46), and premotor and supplementary motor areas (Fig. 2b). Limb apraxia correlated with grey matter loss in the left inferior parietal lobe (Brodmann area 40) (Fig. 2c).
VBM analysis correlating grey matter loss with diadochokinetic rate (apraxia of speech) score (a), orofacial apraxia score (b) and limb apraxia score (c). Statistical parametric maps (SPMs) have been thresholded at p < 0.001 (uncorrected) and rendered on coronal (left), axial (middle) and sagittal (right) sections of a study-specific average group T1-weighted MRI template image in DARTEL space. In coronal and axial sections, the left hemisphere (L) is shown on the left side of the image as indicated. All sagittal sections are through the left hemisphere
Discussion
This study provides further confirmation that PNFA is associated with AOS, and reveals that orofacial apraxia occurs in the majority of cases while limb apraxia occurs in a substantial minority, particularly when there is an associated parkinsonian syndrome. Clinically, these findings suggest a need for some care in equating progressive apraxia with a particular entity such as CBS, and indicate the relevance of assessing patients presenting with PNFA for deficits in the programming of actions beyond speech articulation. The findings further demonstrate specific anatomical substrates for these different forms of apraxia in PNFA: AOS was associated with posterior left inferior frontal gyrus atrophy, orofacial apraxia was associated with atrophy of left middle frontal and premotor cortices, while limb apraxia was associated with more posterior atrophy in the left parietal lobe.
Our findings corroborate previous work, mainly in aphasic stroke, indicating that orofacial apraxia, often though not invariably, accompanies AOS [8, 9, 30]. Anatomically, AOS and orofacial apraxia in this neurodegenerative population showed critical substrates that were in proximity (but non-identical) in these disorders. The neuroanatomical correlates identified here are in keeping with previous evidence [4, 9] and implicate separable mechanisms for the programming of different kinds of complex, learned actions in the left frontal lobe. Clinical and anatomical distinctions between AOS and orofacial apraxia are not absolute and may in part reflect different kinds of actions as well as muscle groups involved in these different forms of apraxia [5, 6]: AOS may represent a deficit of precise sequencing of orofacial and tongue movements, while orofacial apraxia represents a deficit in the execution of discrete (and relatively crude) orofacial actions. Orofacial apraxia may have a more distributed anatomical basis, consistent with a more generic role in orofacial motor control. Our finding that the development of orofacial apraxia, but not AOS or limb apraxia, correlates with disease duration may speak to the anatomical organisation of these functions: strategic damage involving relatively focal cortical modules may be sufficient to produce AOS or limb apraxia, while the more distributed control of relatively simple orofacial movements implies greater neural redundancy but may be correspondingly more vulnerable to cumulative cortical insults with the advancing neurodegenerative process.
Consistent with a large body of clinical observation, we found that limb apraxia was associated with CBS [34–36], however this association was not clinically specific: individual patients with PNFA and no associated parkinsonian features nevertheless exhibited limb apraxia. Anatomically, and in accord with previous anatomical evidence [34–36], limb apraxia was associated with left parietal lobe atrophy. It may be that limb apraxia is an early sign of the development of a parkinsonian syndrome, consistent with previous suggestions of a close pathophysiological relation between these deficits [5]; longitudinal studies of PNFA cohorts will be required to resolve this issue. A further unsettled issue concerns the histopathological substrate for limb apraxia and for the other specific apraxias studied here, and in particular, any specificity for tau versus non-tau inclusions: it has been proposed that AOS (and indeed PNFA more generally) is closely associated with tau pathology, in particular corticobasal degeneration and PSP [17]. This is a further important issue for future longitudinal studies with post mortem correlation.
References
Leiguarda RC, Marsden CD (2000) Limb apraxias: higher-order disorders of sensorimotor integration. Brain 123:860–879
Geschwind N (1965) Disconnexion syndromes in animals and man. I. Brain 88:237–294
Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML (2005) Apraxia of speech: an overview. Neurocase 11:427–432
Raade AS, Rothi LJG, Heilman KM (1991) The relationship between buccofacial and limb apraxia. Brain Cogn 16:130–146
Pramstaller PP, Marsden CD (1996) The basal ganglia and apraxia. Brain 119(Pt 1):319–340
Kimura D, Watson N (1989) The relation between oral movement control and speech. Brain Lang 37(4):565–590
Ackermann H, Riecker A (2004) The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang 89(2):320–328
Dronkers NF (1996) A new brain region for coordinating speech articulation. Nature 384:159–161
Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K (2004) Re-examining the brain regions crucial for orchestrating speech articulation. Brain 127:1479–1487
Mesulam MM (2001) Primary progressive aphasia. Ann Neurol 49:425–432
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL (2004) Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55:335–346
Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL (2008) The logopenic/phonological variant of primary progressive aphasia. Neurology 71:1227–1234
Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML (2007) Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 21(4):S23–S30
Croot K (2002) Diagnosis of AOS: definition and criteria. Semin Speech Lang 23:267–280
Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR Jr, Petersen RC (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129:1385–1398
Josephs KA, Duffy JR (2008) Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol 21:688–692
Graham NL, Bak TH, Hodges JR (2003) Corticobasal degeneration as a cognitive disorder. Mov Disord 18:1224–1232
Pharr V, Uttl B, Stark M, Litvan I, Fantie B, Grafman J (2001) Comparison of apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 56:957–963
Soliveri P, Piacentini S, Girotti F (2005) Limb apraxia in corticobasal degeneration and progressive supranuclear palsy. Neurology 64:448–453
Ozsancak C, Auzou P, Hannequin D (2000) Dysarthria and orofacial apraxia in corticobasal degeneration. Mov Disord 15:905–910
Ozsancak C, Auzou P, Dujardin K, Quinn N, Destée A (2004) Orofacial apraxia in corticobasal degeneration, progressive supranuclear palsy, multiple system atrophy and Parkinson’s disease. J Neurol 251:1317–1323
Tyrrell PJ, Kartsounis LD, Frackowiak RS, Findley LJ, Rossor MN (1991) Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J Neurol Neurosurg Psychiatry 54:351–357
Fuh JL, Liao KK, Wang SJ, Lin KN (1994) Swallowing difficulty in primary progressive aphasia: a case report. Cortex 30:701–705
Sakurai Y, Hashida H, Uesugi H, Arima K, Murayama S, Bando M, Iwata M, Momose T, Sakuta M (1996) A clinical profile of corticobasal degeneration presenting as primary progressive aphasia. Eur Neurol 36:134–137
Sakurai Y, Murayama S, Fukusako Y, Bando M, Iwata M, Inoue K (1998) Progressive aphemia in a patient with Pick’s disease: a neuropsychological and anatomic study. J Neurol Sci 159:156–161
Roth HL, Eskin TA, Kendall DL, Heilman KM (2006) Progressive oculo-orofacial-speech apraxia (POOSA). Neurocase 12:221–227
Joshi A, Roy EA, Black SE, Barbour K (2003) Patterns of limb apraxia in primary progressive aphasia. Brain Cogn 53:403–407
Dabul B (2000) Apraxia battery for adults, Second edn. Pro-Ed, Austin
Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N (2006) Clinical and anatomical correlates of apraxia of speech. Brain Lang 97:343–350
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Ashburner J, Friston KJ (2009) Computing average shaped tissue probability templates. Neuroimage 45:333–341
Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC (2009) Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage 44:99–111
Okuda B, Tachibana H, Kawabata K, Takeda M, Sugita M (1999) Cerebral blood flow correlates of higher brain dysfunctions in corticobasal degeneration. J Geriatr Psychiatry Neurol 12:189–193
Peigneux P, Salmon E, Garraux G, Laureys S, Willems S, Dujardin K, Degueldre C, Lemaire C, Luxen A, Moonen G, Franck G, Destee A, Van der Linden M (2001) Neural and cognitive bases of upper limb apraxia in corticobasal degeneration. Neurology 57:1259–1268
Borroni B, Garibotto V, Agosti C, Brambati SM, Bellelli G, Gasparotti R, Padovani A, Perani D (2008) White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Arch Neurol 65:796–801
Acknowledgments
This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. The Dementia Research Centre is an Alzheimer’s Research Trust Co-ordinating Centre. This work was also funded by the Medical Research Council UK. JDR is supported by a Brain Exit Scholarship. MNR is an NIHR senior investigator. JDW is supported by a Wellcome Trust Intermediate Clinical Fellowship.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rohrer, J.D., Rossor, M.N. & Warren, J.D. Apraxia in progressive nonfluent aphasia. J Neurol 257, 569–574 (2010). https://doi.org/10.1007/s00415-009-5371-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5371-4