Abstract
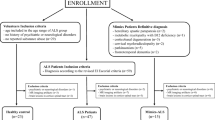
Amyotrophic lateral sclerosis (ALS) is characterised by degeneration of upper (UMN) and lower motor neurons (LMN).We aimed to relate clinical variables to cortical thinning of the primary motor cortex (PMC). The PMC was defined as the region of interest in high-resolution structural MRI scans. We related vertex-wise measures of cortical thinning to UMN involvement, bulbar/limb onset, the total ALS functional rating scale (ALSFRS-R), and its bulbar and upper limb subscore. In total, 93 ALS patients were recruited (60 with classical ALS; 17 with dominant UMN, e.g., primary lateral sclerosis; 16 with pure LMN variant, e.g., progressive muscular atrophy, flail arm or leg syndrome) and compared to 67 age and gender matched healthy controls. The UMN signs in the bulbar regions were associated with bilateral thinning within the bulbar segment on the motor cortex, and UMN signs in spinal regions were associated with thinning in the limb segment of the motor cortex. The site of disease onset (bulbar/lower limb) exhibited the most pronounced thinning in the corresponding part of the motor cortex. According to our analysis, dominant UMN patients demonstrated the most distinct thinning followed by classical ALS patients. Pure LMN variants did not differ from healthy controls. The bulbar subscore of the ALSFRS-R correlated with thinning of the left inferior PMC. Focal morphological changes within the PMC correspond to clinically measured impairments and depend on disease phenotype. Measuring cortical thickness may potentially offer an objective in vivo marker to quantify disease pathology.



Similar content being viewed by others
References
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955. doi:10.1016/S0140-6736(10)61156-7
Brooks BR, Miller RG, Swash M, Munsat TL, Gr WFNR (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Scler Other Motor Neuron Disord 1(5):293–299
Gordon P, Cheng B, Salachas F, Pradat P-F, Bruneteau G, Corcia P, Lacomblez L, Meininger V (2010) Progression in ALS is not linear but is curvilinear. J Neurol 257(10):1713–1717. doi:10.1007/s00415-010-5609-1
Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, Shinoda K, Sugino M, Hanafusa T (2006) Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 66(2):265–267. doi:10.1212/01.wnl.0000194316.91908.8a
Kaufmann P, Levy G, Montes J, Buchsbaum R, Barsdorf AI, Battista V, Arbing R, Gordon PH, Mitsumoto H, Levin B, Thompson JLP, ftQsg (2007) Excellent inter-rater, intra-rater, and telephone-administered reliability of the ALSFRS-R in a multicenter clinical trial. Amyotroph Lateral Sc 8(1):42–46. doi:10.1080/17482960600888156
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A, Grp BAS (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169(1–2):13–21
Ravits JM, La Spada AR (2009) ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology 73(10):805–811. doi:10.1212/WNL.0b013e3181b6bbbd
Ravits J, Paul P, Jorg C (2007) Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 68(19):1571–1575
Turner MR, Brockington A, Scaber J, Hollinger H, Marsden R, Shaw PJ, Talbot K (2010) Pattern of spread and prognosis in lower limb-onset ALS. Amyotroph Lateral Sc 11(4):369–373. doi:10.3109/17482960903420140
Körner S, Kollewe K, Fahlbusch M, Zapf A, Dengler R, Krampfl K, Petri S (2011) Onset and spreading patterns of upper and lower motor neuron symptoms in amyotrophic lateral sclerosis. Muscle Nerve 43(5):636–642. doi:10.1002/mus.21936
Monk PN, Shaw PJ (2006) ALS: life and death in a bad neighborhood. Nat Med 12(8):885–886
van der Graaff MM, de Jong JMBV, Baas F, de Visser M (2009) Upper motor neuron and extra-motor neuron involvement in amyotrophic lateral sclerosis: A clinical and brain imaging review. Neuromuscular Disord 19 (1):53–58. doi:http://dx.doi.org/10.1016/j.nmd.2008.10.002
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N, Hampel H, Gallagher L, Lynch C, Fagan AJ, Pender N, Hardiman O (2012) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2012-302674
Fischl B, Sereno MI, Dale AM (1999) Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. Neuroimage 9 (2):195–207. doi: http://dx.doi.org/10.1006/nimg.1998.0396
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. segmentation and surface reconstruction. Neuroimage 9(2):179–194. doi:10.1006/nimg.1998.0395
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci 97(20):11050–11055. doi:10.1073/pnas.200033797
Kwan JY, Meoded A, Danielian LE, Wu T, Floeter MK (2013) Structural imaging differences and longitudinal changes in primary lateral sclerosis and amyotrophic lateral sclerosis. NeuroImage: Clinical 2 (0):151–160. doi: http://dx.doi.org/10.1016/j.nicl.2012.12.003
Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH (2012) Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 83(4):383–388
Roccatagliata L, Bonzano L, Mancardi G, Canepa C, Caponnetto C (2009) Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotroph Lateral Sc 10(1):47–52
Agosta F, Valsasina P, Riva N, Copetti M, Messina MJ, Prelle A, Comi G, Filippi M (2012) The cortical signature of amyotrophic lateral sclerosis. PLoS ONE 7(8):e42816. doi:10.1371/journal.pone.0042816
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain: J Neurol
Gordon PH, Cheng B, Katz IB, Mitsumoto H, Rowland LP (2009) Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology 72(22):1948–1952. doi:10.1212/Wnl.0b013e3181a8269b
Fischl B, Sereno MI, Tootell RB, Dale AM (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8(4):272–284
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 58(5):695–701. doi:10.1212/wnl.58.5.695
Kuperberg GR, Broome MR, McGuire PK et al (2003) Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiat 60(9):878–888. doi:10.1001/archpsyc.60.9.878
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14(7):721–730. doi:10.1093/cercor/bhh032
Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BTT, Mohlberg H, Amunts K, Zilles K (2008) Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18(8):1973–1980. doi:10.1093/cercor/bhm225
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SCR, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53(5):1051. doi:10.1212/wnl.53.5.1051
Turner MR, Agosta F, Bede P, Govind V, Lulé D, Verstraete E (2012) Neuroimaging in amyotrophic lateral sclerosis. Biomark Med 6(3):319–337. doi:10.2217/bmm.12.26
Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, Floeter MK (2011) White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 134:2642–2655. doi:10.1093/Brain/Awr178
Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH, Matthews PM, Thompson AJ, Smith SM (2009) Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 30(2):615–624. doi:10.1002/hbm.20527
Acknowledgments
We are thankful to Maria Veit for assistance in data acquisition.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schuster, C., Kasper, E., Machts, J. et al. Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: a neuroimaging study. J Neurol 260, 2856–2864 (2013). https://doi.org/10.1007/s00415-013-7083-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7083-z