Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that still lacks reliable diagnostic biomarkers. This study aims to evaluate the diagnostic and prognostic potential of CSF total Tau (t-Tau), phospho-Tau (p-Tau) and p-Tau/t-Tau ratio in ALS patients using CSF neurofilament light (NFL) as the reference biomarker.
Methods
Eighty-five incident ALS, 30 ALS-mimicking (AM) diseases and 51 other non-neurodegenerative diseases (ONND) were included in the study.
Results
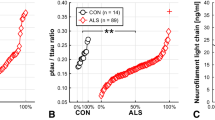
ALS patients had higher levels of CSF t-Tau and lower p-Tau/t-Tau ratio than AM (p = 0.005 and p = 0.006) and ONND (p < 0.001). CSF t-Tau levels discriminated ALS from AM with a sensitivity of 69% and specificity of 60%, and from ONND with a sensitivity of 88% and specificity of 51%. These values were lower than the accuracy of CSF NFL in ALS (sensitivity 86% and specificity 87% in distinguishing ALS from AM and sensitivity 83% and specificity 75% from ONND); CSF t-Tau correlated with progression rate and SNIP. CSF p-Tau did not show relation with any ALS clinical features. CSF NFL significantly correlated with all considered clinical parameters. High levels of CSF t-Tau and NFL were related to poor survival.
Conclusion
CSF t-Tau showed no reliable diagnostic significance but the relation between the high levels of CSF t-Tau and short survival suggests the potential prognostic role of this biomarker in ALS. However, CSF NFL was confirmed to be the most reliable and efficient tool for diagnosis and prediction of clinical progression and survival in ALS patients.



Similar content being viewed by others
References
Strong M, Rosenfeld J (2003) Amyotrophic lateral sclerosis: a review of current concepts. Amyotroph Lateral Scler Other Motor Neuron Disord 4(3):136–143
Chiò A, Logroscino G, Traynor BJ et al (2013) Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 41(2):118–130
Chia R, Chiò A, Traynor BJ (2018) Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol 17(1):94–102
Van den Berg-Vos RM, Visser J, Franssen H et al (2003) Sporadic lower motor neuron disease with adult onset: classification of subtypes. Brain 126(Pt 5):1036–1047
Chiò A, Calvo A, Moglia C, Mazzini L, Mora G, PARALS study group (2011) Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 82(7):740–746
Turner MR, Kiernan MC, Leigh PN, Talbot K (2009) Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol 8(1):94–109
Bowser R, Turner MR, Shefner J (2011) Biomarkers in amyotrophic lateral sclerosis: opportunities and limitations. Nat Rev Neurol 7(11):631–638
Tarasiuk J, Kułakowska A, Drozdowski W, Kornhuber J, Lewczuk P (2012) CSF markers in amyotrophic lateral sclerosis. J Neural Transm 119(7):747–757
Tortelli R, Ruggieri M, Cortese R et al (2012) Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur J Neurol 19(12):1561–1567
Tortelli R, Copetti M, Ruggieri M et al (2015) Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol 22(1):215–218
Steinacker P, Feneberg E, Weishaupt J et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20
Poesen K, De Schaepdryver M, Stubendorff B et al (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88(24):2302–2309
Süssmuth SD, Tumani H, Ecker D, Ludolph AC (2003) Amyotrophic lateral sclerosis: disease stage related changes of tau protein and S100 beta in cerebrospinal fluid and creatine kinase in serum. Neurosci Lett 353(1):57–60
Jiménez-Jiménez FJ, Hernánz A, Medina-Acebrón S et al (2005) Tau protein concentrations in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neurol Scand 111(2):114–117
Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H (2006) Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66(6):852–856
Paladino P, Valentino F, Piccoli T, Piccoli F, La Bella V (2009) Cerebrospinal fluid tau protein is not a biological marker in amyotrophic lateral sclerosis. Eur J Neurol 16(2):257–261
Goedert M (1993) Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci 16:460–465
Süssmuth SD, Reiber H, Tumani H (2001) Tau protein in cerebrospinal fluid (CSF): a blood-CSF barrier related evaluation in patients with various neurological diseases. Neurosci Lett 300(2):95–98
Sunderland T, Linker G, Mirza N et al (2003) Decreased beta amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 289:2094–2103
Satoh K, Shirabe S, Tsujino A et al (2007) Total tau protein in cerebrospinal fluid and diffusion-weighted MRI as an early diagnostic marker for Creutzfeldt–Jakob disease. Dement Geriatr Cogn Disord 24:207–212
Grossman M, Elman L, McCluskey L et al (2014) Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol 2014;71(4):442–448
Wilke C, Deuschle C, Rattay TW, Maetzler W, Synofzik M (2015) Total tau is increased, but phosphorylated tau not decreased, in cerebrospinal fluid in amyotrophic lateral sclerosis. Neurobiol Aging 36(2):1072–1074
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299
Strong MJ, Abrahams S, Goldstein LH et al (2017) Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18(3–4):153–174
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1–2):13–21
Aitkens S, Lord J, Bernauer E et al (1989) Relationship of manual muscle testing to objective strength measurements. Muscle Nerve 12:173–177
Tortelli R, Copetti M, Panza F et al (2016) Time to generalisation as a predictor of prognosis in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 87(6):678–679
Kimura F, Fujimura C, Ishida S et al (2006) Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 66:265–267
Miller JM, Moxham J, Green M (1985) The maximal sniff in the assessment of diaphragm function in man. Clin Sci 69:91–96
Woolley SC, York MK, Moore DH et al (2010) Detecting frontotemporal dysfunction in ALS: utility of the ALS cognitive behavioral screen (ALS-CBS). Amyotroph Lateral Scler 11(3):303–311
Poletti B, Solca F, Carelli L et al (2016) The validation of the Italian Edinburgh cognitive and behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener 17(7–8):489–498
Goldstein ME, Sternberger NH, Sternberger LA (1987) Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol 14(2):149–160
Pijnenburg YA, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE (2015) Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimer’s Dement 1(4):505–512
Petzold A, Altintas A, Andreoni L et al (2010) Neurofilament ELISA validation. J Immunol Methods 352(1–2):23–31
Bourbouli M, Rentzos M, Bougea A et al (2017) Cerebrospinal fluid TAR DNA-binding protein 43 combined with Tau proteins as a candidate biomarker for amyotrophic lateral sclerosis and frontotemporal dementia spectrum disorders. Dement Geriatr Cogn Disord 44(3–4):144–152
Ganesalingam J, An J, Shaw CE et al (2011) Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem 117(3):528–537
Lehnert S, Costa J, de Carvalho M et al (2014) Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 15(5–6):344–350
Labra J, Menon P, Byth K et al (2016) Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry 87(6):628–632
Chiò A, Logroscino G, Hardiman O et al (2009) Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 10(5–6):310–323
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Scarafino, A., D’Errico, E., Introna, A. et al. Diagnostic and prognostic power of CSF Tau in amyotrophic lateral sclerosis. J Neurol 265, 2353–2362 (2018). https://doi.org/10.1007/s00415-018-9008-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9008-3