Abstract
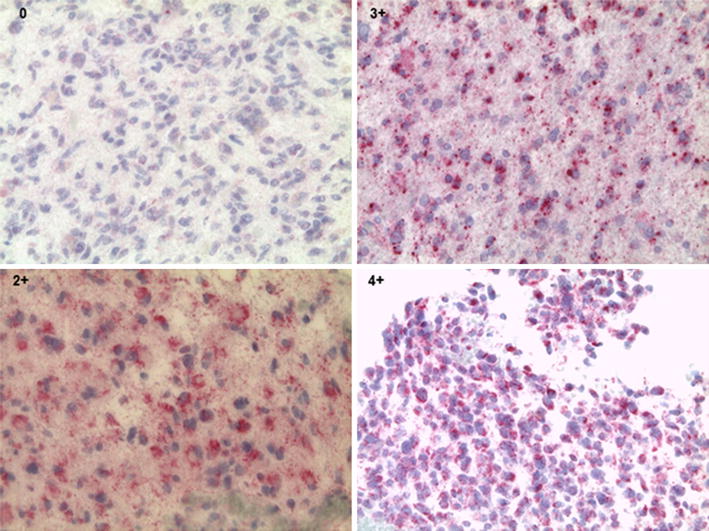
Glioblastoma multiforme (GBM) is a highly lethal brain tumor affecting children and adults, with the majority of affected individuals dying from their disease by 2 years following diagnosis. Other groups have reported the association of cytomegalovirus (CMV) with GBM, and we sought to confirm these findings in a large series of patients with primary GBM from our institution. Immunohistochemical analysis of paraffin embedded tissue sections was performed on 49 newly diagnosed GBM tumors, the largest series reported to date. We confirmed the presence of CMV pp65 on 25/49 (51%) and of IE1 on 8/49 (16%) of these tumors. While pp65 and IE1 are generally found in the nucleus of cells that are permissibly infected by CMV, GBM in this series had mostly cytoplasmic staining, with only 16% having nuclear staining for one or both of these antigens. We infected GBM cell lines with a laboratory strain of CMV, and found that most of the staining was cytoplasmic, with some perinuclear localization of IE1. To test the potential for CMV infected GBM cells to be recognized by CMV pp65 and IE1 specific cytotoxic T lymphocytes (CTL), we used CMV infected GBM cell lines in cytotoxicity assays with human leukocyte antigen partially matched CMV CTL. Lysis of CMV infected GBM tumor cells was accentuated by pre-treating these cell lines with either the demethylating agent decitabine or interferon-γ, both of which were shown to increase MHC Class I and II expression on tumor cells in vitro. These studies confirm the presence of CMV pp65 or IE1 on approximately half of GBM, with the possibility that CMV positive tumor cells can be recognized by CMV pp65/IE1 specific T cells.




Similar content being viewed by others
References
Davis FG et al (2001) Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol 3(3):152–158
Surawicz TS et al (1998) Brain tumor survival: results from the National Cancer Data Base. J Neurooncol 40(2):151–160
Zhang JG et al (2007) Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res 13(2 Pt 1):566–575
Saikali S et al (2007) Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol 81(2):139–148
Sampson JH et al (1996) Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci USA 93(19):10399–10404
Yu JS et al (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61(3):842–847
Mitchell DA, Fecci PE, Sampson JH (2008) Immunotherapy of malignant brain tumors. Immunol Rev 222:70–100
Liau LM et al (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11(15):5515–5525
Merchant RE et al (1988) Intralesional infusion of lymphokine-activated killer (LAK) cells and recombinant interleukin-2 (rIL-2) for the treatment of patients with malignant brain tumor. Neurosurgery 23(6):725–732
Lillehei KO et al (1991) Long-term follow-up of patients with recurrent malignant gliomas treated with adjuvant adoptive immunotherapy. Neurosurgery 28(1):16–23
Dillman RO et al (2004) Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 27(5):398–404
Serraino D et al (2005) Infection with Epstein-Barr virus and cancer: an epidemiological review. J Biol Regul Homeost Agents 19(1–2):63–70
Eiben GL et al (2003) Cervical cancer vaccines: recent advances in HPV research. Viral Immunol 16(2):111–121
Bosch FX et al (2005) Epidemiology of hepatocellular carcinoma. Clin Liver Dis 9(2):191–211, v
Vilchez RA, Butel JS (2003) Simian virus 40 and its association with human lymphomas. Curr Oncol Rep 5(5):372–379
Harkins L et al (2002) Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360(9345):1557–1563
Cobbs CS et al (2002) Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62(12):3347–3350
Samanta M et al (2003) High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol 170(3):998–1002
Mitchell DA et al (2008) Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol 10(1):10–18
Scheurer ME et al (2008) Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol 116(1):79–86
Poltermann S et al (2006) Lack of association of herpesviruses with brain tumors. J Neurovirol 12(2):90–99
Lau SK et al (2005) Lack of association of cytomegalovirus with human brain tumors. Mod Pathol 18(6):838–843
Cinatl J et al (2008) Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for a viral infection in tumor progression. FEMS Microbiol Rev 28:59–77
Hagemeier C et al (1994) Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J 13(12):2897–2903
Fortunato EA et al (1997) Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J Virol 71(11):8176–8185
Tsai HL et al (1996) Human cytomegalovirus immediate-early protein IE2 tethers a transcriptional repression domain to p53. J Biol Chem 271(7):3534–3540
Bao L et al (2008) Expansion of cytomegalovirus pp65 and IE-1 specific cytotoxic T lymphocytes for cytomegalovirus-specific immunotherapy following allogeneic stem cell transplantation. Biol Blood Marrow Transplant 14(10):1156–1162
Sun Q et al (2000) B lymphoblastoid cell lines as efficient APC to elicit CD8+ T cell responses against a cytomegalovirus antigen. J Immunol 165(7):4105–4111
Yang I et al (2004) Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex-bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg 100(2):310–319
Read SB et al (2003) Human alloreactive CTL interactions with gliomas and with those having upregulated HLA expression from exogenous IFN-gamma or IFN-gamma gene modification. J Interferon Cytokine Res 23(7):379–393
Serrano A et al (2001) Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer 94(2):243–251
Ogura T et al (1986) Human cytomegalovirus persistent infection in a human central nervous system cell line: production of a variant virus with different growth characteristics. J Gen Virol 67(Pt 12):2605–2616
Luo MH, Fortunato EA (2007) Long-term infection and shedding of human cytomegalovirus in T98G glioblastoma cells. J Virol 81(19):10424–10436
Sabatier J et al (2005) Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer 92(4):747–750
Staras SA et al (2006) Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 43(9):1143–1151
Boeke CE et al (2008) CMV antibody prevalence and seroincidence in plateletpheresis donors. J Clin Apher 23(2):63–65
Thurner B et al (1999) Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 190(11):1669–1678
Coulie PG et al (2002) Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev 188:33–42
Ho WY et al (2006) In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods 310(1–2):40–52
Borysiewicz LK et al (1988) Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med 168(3):919–931
Liu YN, Kari B, Gehrz RC (1988) Human immune responses to major human cytomegalovirus glycoprotein complexes. J Virol 62(3):1066–1070
Ploegh HL (1998) Viral strategies of immune evasion. Science 280(5361):248–253
Oshiro S et al (2000) Response of MHC class-1 antigen on rat glioma cells to cytokines. Anticancer Res 20(1C):605–610
Piguet V et al (1986) Heterogeneity of the induction of HLA-DR expression by human immune interferon on glioma cell lines and their clones. J Natl Cancer Inst 76(2):223–228
Wen PY, Lampson MA, Lampson LA (1992) Effects of gamma-interferon on major histocompatibility complex antigen expression and lymphocytic infiltration in the 9L gliosarcoma brain tumor model: implications for strategies of immunotherapy. J Neuroimmunol 36(1):57–68
Mitchell D et al (2008) Efficacy of a phase II vaccine targeting cytomegalovirus antigens in newly-diagnosed GBM. J Clin Oncol 26:2042
Prins RM, Cloughesy TF, Liau LM (2008) Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med 359(5):539–541
Bao L, Sun Q, Lucas KG (2007) Rapid generation of CMV pp65-specific T cells for immunotherapy. J Immunother (1997) 30(5):557–561
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucas, K.G., Bao, L., Bruggeman, R. et al. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neurooncol 103, 231–238 (2011). https://doi.org/10.1007/s11060-010-0383-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0383-6