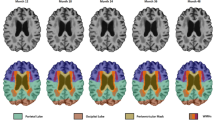
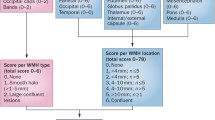
Abstract
As the older adult segment of the population increases, Alzheimer’s disease (AD) has emerged as a significant public health epidemic. Over the past 3 decades, advances in the understanding of the biology of AD have led to a somewhat unified hypothesis of disease pathogenesis that emphasizes the precipitating role of beta amyloid protein. However, several lines of evidence suggest that multiple pathologies are necessary for clinical manifestation of the disease. Our focus over the past several years has been on the contribution of small vessel cerebrovascular disease, visualized as white matter hyperintensities (WMH) on magnetic resonance imaging, to AD. White matter hyperintensity volume, particularly in parietal regions, is elevated among individuals with and at risk for AD, predicts future diagnosis of AD, predicts the rate of progression of cognitive symptoms among individuals with AD, and increases over time among individuals destined to develop AD. White matter hyperintensities may represent an independent source of impairment and/or may interact more fundamentally with “primary” AD pathology. Future work should focus on more inclusive models of that better define “normal” vs “pathological” aging.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Jack Jr CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. This paper presents a comprehensive hypothesis regarding the cascade of biological events that contribute to the pathogensis of AD.
• Jack Jr CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. This paper is an updated version of a pathogenic model for Alzheimer’s disease.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2010;7:280–92.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2010;7:270–9.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2010;7:263–9.
Sperling R, Donohue M, Aisen P. The A4 trial: anti-amyloid treatment of asymptomatic Alzheimer’s disease. Alzheimers Dement. 2012;8(Suppl):425–6.
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17.
Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52.
Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–5.
Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130(Pt 10):2607–15.
Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, et al. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–44.
Rodrigue KM, Kennedy KM, Devous Sr MD, Rieck JR, Hebrank AC, Diaz-Arrastia R, et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–95.
Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–7.
Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94.
Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–23.
Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(Pt 11):2837–44.
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83.
Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–81.
Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7.
Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–42.
Pavlopoulos E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, et al. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Sci Transl Med. 2013;5:200ra115.
Brickman AM, Stern Y, Small SA. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21:923–8.
Castellani RJ, Perry G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: the flat line of progress. Arch Med Res. 2012;43:694–8.
Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(Pt 9):2697–706.
Elkins JS, O’Meara ES, Longstreth Jr WT, Carlson MC, Manolio TA, Johnston SC. Stroke risk factors and loss of high cognitive function. Neurology. 2004;63:793–9.
Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–6.
Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–9.
Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ (Clinical research ed). 2001;322:1447–51.
Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–8.
Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–8.
Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51.
Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92.
Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41.
Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–93.
Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–8.
Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28.
Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42:2672–713.
Kertesz A, Black SE, Tokar G, Benke T, Carr T, Nicholson L. Periventricular and subcortical hyperintensities on magnetic resonance imaging. Rims, caps, and unidentified bright objects. Arch Neurol. 1988;45:404–8. Arch Neurol. 1988;45:404–8.
Román GC. Senile dementia of the binswanger type: a vascular form of dementia in the elderly. JAMA. 1987;258:1782–8.
DeCarli C, Murphy DG, Tranh M, Grady CL, Haxby JV, Gillette JA, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–84.
Bronge L, Wahlund LO. White matter changes in dementia: does radiology matter? Br J Radiol. 2007;80:S115–20.
Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, et al. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81:977–83.
Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80.
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9.
Launer LJ. Epidemiology of white matter lesions. Top Magn Reson Imaging. 2004;15:365–7.
Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriat Psychiatry. 2009;24:109–17.
Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32.
Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Alzheimer’s disease: do white matter hyperintensities matter? Dialog Clin Neurosci. 2009;11:181–90.
Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12.
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR. 1987;149:351–6.
Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, et al. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res. 2011;193:101–6.
Admiraal-Behloul F, Olofesen H, Van den Heuvel DM, Schmitz N, Reiber JH, Van Buchem MA. Fully automated lobe delineation for regional white matter lesion load quantification in a large scale study. Proc Int Soc Magnet Reson Med. 2004:138.
• Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;18:1–7. In this paper we showed that both WMH and amyloid are associated with Alzheimer’s disease and that among individuals with evidence of cerebral amyloidosis, those with higher WMH burden were more likely to manifest clinical symptoms.
Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid an white matter lesions in AD, CI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–9.
Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of Alzheimer’s disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56.
Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, et al. Brain morphology in older African Americans, Caribbean Hispanics, and Whites from northern Manhattan. Arch Neurol. 2008;65:1053–61.
Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain. 1992;115(Pt 3):735–48.
Kalaria RN. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging. 2000;21:321–30.
Rezek DL, Morris JC, Fulling KH, Gado MH. Periventricular white matter lucencies in senile dementia of the Alzheimer type and in normal aging. Neurology. 1987;37:1365–8.
Luchsinger JA, Brickman AM, Reitz C, Cho SJ, Schupf N, Manly JJ, et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009;73:450–6.
Brickman AM, Provenzano FA, Muraskin J, Guzman VA, Manly JJ, Schupf N, et al. Distribution of MRI-defined white matter hyperintensities in mild cognitive impairment [abstract]. J Int Neuropsychol Soc. 2011;17(S1).
Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Demen Geriat Cognit Dis. 2004;18:180–8.
Meier IB, Manly JJ, Provenzano FA, Hector J, Wasserman BT, Louie K, et al. White matter predictors of cogntiive functioning in older adults. Annual meeting of the International Neuropsychological Society; February, 2011; Boston, MA 2011.
Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–8.
Leys D, Pruvo JP, Parent M, Vermersch P, Soetaert G, Steinling M, et al. Could Wallerian degeneration contribute to “leuko-araiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry. 1991;54:46–50.
Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–4.
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22.
Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriat Psychiat. 2000;15:803–12.
Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100.
Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69:1621–7.
Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65:1202–8.
Wiegman AF, Meier IB, Provenzano FA, Schupf N, Manly JJ, Stern Y, et al. Regional white matter hyperintensity volume and cognition predict death in a multi-ethnic, community cohoort of older adults. J Am Geriatr Soc. 2013.
Brickman AM, Zarhodne LB, Guzman VA, Narkhede A, Provenzano FA, Schupf N, et al. Reconsidering harbingers of Alzheimer’s disease: regionally distributed progression of white matter hyperintensities in the community. [In preparation].
Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–9.
Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest Heart Fail. 2013;19:E29–34.
Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, et al. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens. 2013;7:336–43.
Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–8.
Portet F, Brickman AM, Stern Y, Scarmeas N, Muraskin J, Provenzano FA, et al. Metabolic syndrome and localization of white matter hyperintensities in the elderly population. Alzheimers Dement. 2012;8(5 Suppl):S88–95 e1.
Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–20.
Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–33.
Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebral auotregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–23.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:14052–7.
Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9:112–22.
Weller RO, Cohen NR, Nicoll JA. Cerebrovascular disease and the pathophysiology of Alzheimer’s disease. Implications for therapy. Panminerva Med. 2004;46:239–51.
Niwa K, Carlson GA, Iadecola C. Exogenous A beta1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–68.
Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–17.
Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–71.
Maia LF, Vasconcelos C, Seixas S, Magalhaes R, Correia M. Lobar brain hemorrhages and white matter changes: clinical, radiological and laboratorial profiles. Cerebrovasc Dis. 2006;22:155–61.
Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–7.
Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–5.
Meier IB, Narkhede A, Provenzano FA, Luchsinger JA, Manly JJ, Willey JZ, et al. Lobar microbleeds are associated with white matter hyperintensities and memory in older adults [abstract]. J Int Neuropsychol Soc. 2012;18:224–5.
Guzman VA, Carmichael OT, Schwarz C, Tosto G, Zimmerman ME, Brickman AM, et al. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume. Alzheimer’s and Dementia. 2013;9:S124–31.
van der Flier WM, Barkhof F, Scheltens P. Shifting paradigms in dementia: toward stratification of diagnosis and treatment using MRI. Ann NY Acad Sci. 2007;1097:215–24.
Provenzano FA, Cortes ER, Dashnaw S, Brickman AM. Neuroimaging-guided pathological examination of white matter hyperintensities in aging. Annual Meeting of the International Neuropsychological Society; February, 2011; Boston, MA. 2011.
Acknowledgment
Work presented here was supported in part by grants from the National Institutes of Health (AG034189, AG037212, AG028786, AG029949, AG024708), Alzheimer’s Association, Mary E. Groff Surgical Medical Research and Education Charitable Trust, and Columbia University.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Adam M. Brickman has received travel/accommodations expenses covered or reimbursed from the International Neuropsychological Society (as a board member) and the Alzheimer's Association.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical collection on Dementia
Rights and permissions
About this article
Cite this article
Brickman, A.M. Contemplating Alzheimer’s Disease and the Contribution of White Matter Hyperintensities. Curr Neurol Neurosci Rep 13, 415 (2013). https://doi.org/10.1007/s11910-013-0415-7
Published:
DOI: https://doi.org/10.1007/s11910-013-0415-7