Abstract
Introduction
Cerebral vasoconstriction is associated with increased cytosolic Ca2+ concentration in vascular smooth muscle, presumably due to Ca2+ influx and Ca2+ release from intracellular stores. We tested the hypothesis that dantrolene (a blocker of Ca2+-induced Ca2+ release from the ryanodine receptor channel on the sarco-endoplasmic reticulum) would potentiate the action of nimodipine (a voltage-dependent L-type Ca2+ channel blocker, considered standard therapy for SAH) in inhibiting the vasoconstriction of isolated cerebral arteries.
Method
Sprague–Dawley rat basilar and femoral arteries were analyzed for ryanodine receptor expression by immunofluorescence and PCR. Vasoconstriction of basilar artery ex vivo was measured in a wire myograph while exposed to serotonin (5-HT) or endothelin-1 (ET-1) in the presence or absence of dantrolene (10–100 μM) and/or nimodipine (30 nM). Femoral artery was examined for comparison.
Results
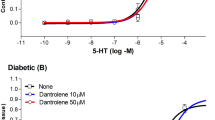
Basilar and femoral arteries express only the ryanodine receptor 3 (RyR3) isoform. In both basilar and femoral arteries, dantrolene significantly inhibited the constriction to 5-HT, whereas it poorly affected the constriction to ET-1. The inhibitory effect of dantrolene on 5-HT was substantially increased by nimodipine, inducing a 10-fold increase in the 50% effective concentration of 5-HT and a 46% reduction in maximum basilar constriction. In femoral artery, dantrolene modestly affected constriction to phenylephrine and there was no interaction with nimodipine.
Conclusion
Dantrolene has synergistic effects with nimodipine against 5-HT-induced vasoconstriction in isolated cerebral arteries. Dantrolene–nimodipine interaction will require testing in a pathophysiological model but might provide treatment for reducing SAH-related vasospasm or other 5-HT-related vasospastic syndromes, such as Call-Fleming syndrome.






Similar content being viewed by others
References
Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene—a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–73. doi:10.1111/j.1365-2044.2004.03658.x.
Endo M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J Pharmacol Sci. 2006;100(5):519–24. doi:10.1254/jphs.CPJ06004X.
Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem. 2001;276(17):13810–6.
Dorsch NW. Cerebral arterial spasm—a clinical review. Br J Neurosurg. 1995;9(3):403–12. doi:10.1080/02688699550041403.
Tani E, Matsumoto T. Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol. 2004;2(1):13–21. doi:10.2174/1570161043476492.
Beg SS, Hansen-Schwartz JA, Vikman PJ, Xu CB, Edvinsson LI. Protein kinase C inhibition prevents upregulation of vascular ET(B) and 5-HT(1B) receptors and reverses cerebral blood flow reduction after subarachnoid haemorrhage in rats. J Cereb Blood Flow Metab. 2007;27(1):21–32. doi:10.1038/sj.jcbfm.9600313.
Ansar S, Svendgaard NA, Edvinsson L. Neurokinin-1 receptor antagonism in a rat model of subarachnoid hemorrhage: prevention of upregulation of contractile ETB and 5-HT1B receptors and cerebral blood flow reduction. J Neurosurg. 2007;106(5):881–6. doi:10.3171/jns.2007.106.5.881.
Alafaci C, Jansen I, Arbab MA, Shiokawa Y, Svendgaard NA, Edvinsson L. Enhanced vasoconstrictor effect of endothelin in cerebral arteries from rats with subarachnoid haemorrhage. Acta Physiol Scand. 1990;138(3):317–9.
Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;(3):CD000277.
Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38(4):321–416.
Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger – cyclic ADP-ribose. J Cell Mol Med. 2006;10(2):407–22. doi:10.1111/j.1582-4934.2006.tb00408.x.
Pacaud P, Loirand G. Release of Ca2+ by noradrenaline and ATP from the same Ca2+ store sensitive to both InsP3 and Ca2+ in rat portal vein myocytes. J Physiol. 1995;484(Pt 3):549–55.
Kamishima T, McCarron JG. Regulation of the cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from rat cerebral arteries. J Physiol. 1997;501(Pt 3):497–508. doi:10.1111/j.1469-7793.1997.497bm.x.
Asano M, Kuwako M, Nomura Y, et al. Possible mechanism of the potent vasoconstrictor responses to ryanodine in dog cerebral arteries. Eur J Pharmacol. 1996;311(1):53–60. doi:10.1016/0014-2999(96)00408-6.
Loch Macdonald R. Management of cerebral vasospasm. Neurosurg Rev. 2006;29(3):179–93. doi:10.1007/s10143-005-0013-5.
Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care. 2008. doi:10.1007/s12028-008-9133-4.
Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41(1):19–26.
Ramsch KD, Ahr G, Tettenborn D, Auer LM. Overview on pharmacokinetics of nimodipine in healthy volunteers and in patients with subarachnoid hemorrhage. Neurochirurgia (Stuttg). 1985;28 Suppl 1:74–8.
Schilling L, Vatter H, Mursch K, Ehrenreich H, Schmiedek P. Characterization of the contractile and relaxant action of the endothelin-1 precursor, big endothelin-1, in the isolated rat basilar artery. Peptides. 2000;21(1):91–9. doi:10.1016/S0196-9781(99)00179-5.
Salomone S, Morel N, Godfraind T. Role of nitric oxide in the contractile response to 5-hydroxytryptamine of the basilar artery from Wistar Kyoto and stroke-prone rats. Br J Pharmacol. 1997;121(6):1051–8. doi:10.1038/sj.bjp.0701227.
Nishimura Y. Characterization of 5-hydroxytryptamine receptors mediating contractions in basilar arteries from stroke-prone spontaneously hypertensive rats. Br J Pharmacol. 1996;117(6):1325–33.
Jackowski A, Crockard A, Burnstock G, Lincoln J. Alterations in serotonin and neuropeptide Y content of cerebrovascular sympathetic nerves following experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1989;9(3):271–9.
Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34(5):1269–75. doi:10.1161/01.STR.0000065829.45234.69.
Chang JY, Hardebo JE, Owman C. Differential vasomotor action of noradrenaline, serotonin, and histamine in isolated basilar artery from rat and guinea-pig. Acta Physiol Scand. 1988;132(1):91–102.
Kitazono T, Faraci FM, Heistad DD. Effect of norepinephrine on rat basilar artery in vivo. Am J Physiol. 1993;264(1 Pt 2):H178–82.
Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111(3):674–706. doi:10.1016/j.pharmthera.2005.12.004.
Villalon CM, Centurion D. Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol. 2007;376(1–2):45–63. doi:10.1007/s00210-007-0179-1.
Parsons AA, Whalley ET. Evidence for the presence of 5-HT1-like receptors in rabbit isolated basilar arteries. Eur J Pharmacol. 1989;174(2–3):189–96. doi:10.1016/0014-2999(89)90311-7.
Parsons AA, Whalley ET, Feniuk W, Connor HE, Humphrey PP. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br J Pharmacol. 1989;96(2):434–40.
Bae YM, Kim A, Kim J, et al. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347(2):468–76. doi:10.1016/j.bbrc.2006.06.116.
Bae YM, Kim A, Lee YJ, et al. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2007;25(4):809–17. doi:10.1097/HJH.0b013e3280148312.
MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569(Pt 2):533–44. doi:10.1113/jphysiol.2005.096529.
Sakurada S, Okamoto H, Takuwa N, Sugimoto N, Takuwa Y. Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am J Physiol Cell Physiol. 2001;281(2):C571–8.
Kawanabe Y, Nauli SM. Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function. Cell Signal. 2005;17(8):911–6. doi:10.1016/j.cellsig.2005.01.001.
Kawanabe Y, Masaki T, Hashimoto N. Involvement of phospholipase C in endothelin 1-induced stimulation of Ca++ channels and basilar artery contraction in rabbits. J Neurosurg. 2006;105(2):288–93. doi:10.3171/jns.2006.105.2.288.
Evans AM, Cobban HJ, Nixon GFET. (A) receptors are the primary mediators of myofilament calcium sensitization induced by ET-1 in rat pulmonary artery smooth muscle: a tyrosine kinase independent pathway. Br J Pharmacol. 1999;127(1):153–60. doi:10.1038/sj.bjp.0702548.
Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998;164(4):437–48. doi:10.1046/j.1365-201X.1998.00454.x.
Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1-induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol. 2002;283(3):H983–9.
Scherer EQ, Herzog M, Wangemann P. Endothelin-1-induced vasospasms of spiral modiolar artery are mediated by rho-kinase-induced Ca(2+) sensitization of contractile apparatus and reversed by calcitonin gene-related peptide. Stroke. 2002;33(12):2965–71. doi:10.1161/01.STR.0000043673.22993.FD.
Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 2001;20(11):2666–71. doi:10.1093/emboj/20.11.2666.
Higashida H. ADP-ribosyl cyclase coupled with receptors via G proteins. FEBS Lett. 1997;418(3):355–6. doi:10.1016/S0014-5793(97)01410-5.
Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. a primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem. 2001;276(14):11180–8. doi:10.1074/jbc.M004849200.
Godfraind T. Calcium-channel modulators for cardiovascular disease. Expert Opin Emerg Drugs. 2006;11(1):49–73. doi:10.1517/14728214.11.1.49.
Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56(4):439–513. doi:10.1124/pr.56.4.1.
Takeshima H, Ikemoto T, Nishi M, et al. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem. 1996;271(33):19649–52. doi:10.1074/jbc.271.33.19649.
Lohn M, Jessner W, Furstenau M, et al. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89(11):1051–7. doi:10.1161/hh2301.100250.
Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508(Pt 1):211–21.
Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113(2):229–38. doi:10.1085/jgp.113.2.229.
Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112(5):717–24.
Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115(5):653–62. doi:10.1085/jgp.115.5.653.
Hansen-Schwartz J, Ansar S, Edvinsson L. Cerebral vasoconstriction after subarachnoid hemorrhage—role of changes in vascular receptor phenotype. Front Biosci. 2008;13:2160–4. doi:10.2741/2831.
Mendelow AD, McCalden TA, Hattingh J, Coull A, Rosendorff C, Eidelman BH. Cerebrovascular reactivity and metabolism after subarachnoid hemorrhage in baboons. Stroke. 1981;12(1):58–65.
Sahlin C, Owman C, Chang JY, Delgado T, Salford LG, Svendgaard NA. Changes in contractile response and effect of a calcium antagonist, nimodipine, in isolated intracranial arteries of baboon following experimental subarachnoid hemorrhage. Brain Res Bull. 1990;24(3):355–61. doi:10.1016/0361-9230(90)90089-I.
Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–44.
Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurg Focus. 2006;21(3):E8. doi:10.3171/foc.2006.21.3.8.
Dodick DW. Reversible segmental cerebral vasoconstriction (Call-Fleming syndrome): the role of calcium antagonists. Cephalalgia. 2003;23(3):163–5. doi:10.1046/j.1468-2982.2003.00506.x.
Muehlschlegel S, Rordorf G, Bodock M, Sims JR. Dantrolene mediates vasorelaxation in cerebral vasoconstriction: a case series. Neurocrit Care. 2008. doi:10.1007/s12028-008-9132-5.
Acknowledgment
Dr. John Sims is supported by NIH 1 K08 NS049241-01A2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salomone, S., Soydan, G., Moskowitz, M.A. et al. Inhibition of Cerebral Vasoconstriction by Dantrolene and Nimodipine. Neurocrit Care 10, 93–102 (2009). https://doi.org/10.1007/s12028-008-9153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9153-0