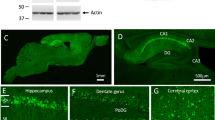
Abstract
We investigated synaptic communication and plasticity in hippocampal slices from mice overexpressing mutated 695-amino-acid human amyloid precursor protein (APP695SWE), which show behavioral and histopathological abnormalities simulating Alzheimer's disease. Although aged APP transgenic mice exhibit normal fast synaptic transmission and short term plasticity, they are severely impaired in in-vitro and in-vivo long-term potentiation (LTP) in both the CA1 and dentate gyrus regions of the hippocampus. The LTP deficit was correlated with impaired performance in a spatial working memory task in aged transgenics. These deficits are accompanied by minimal or no loss of presynaptic or postsynaptic elementary structural elements in the hippocampus, suggesting that impairments in functional synaptic plasticity may underlie some of the cognitive deficits in these mice and, possibly, in Alzheimer's patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Citron, M. et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature 360 , 672–674 (1992).
Cai, X., Golde, T. E. & Younkin, S. G. Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science 259, 514–516 (1993).
Suzuki, N. et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science 264, 1335–1340 ( 1994).
Borchelt, D. R. et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ration in vitro and in vivo . Neuron 17, 1005–1013 (1996).
Duff, K. et al. Increased amyloid-beta 42(43) in brains of mice expressing presenilin 1. Nature 383, 710–713 (1996).
Goate, A. M. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706 (1991).
Mullan, M. et al. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of β-amyloid. Nat. Genet. 1, 345–347 ( 1992).
Chartier-Harlin, M. C. et al. Early-onset Alzheimer's disease caused by mutation at codon 717 of the β-amyloid precursor protein gene. Nature 353, 844–846 (1991).
Murrell, J., Farlow, M., Getti, B. & Benson, M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science 254, 97–99 (1991).
Hendriks, L. et al. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the β-amyloid precursor protein gene. Nat. Genet. 1, 218–221 ( 1992).
Eckman, C. B. et al. A new pathogenic mutation in the APP gene (I716V) increases the relative proportion of A-beta 42(43). Hum. Mol. Genet. 6, 2087–2089 (1997).
Hardy, J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 20, 154–159 ( 1997).
Hsiao, K. K. et al. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Irizarry, M. C., McNamara, M., Fedorchak, K., Hsiao, K. K. & Hyman, B. T. APPSW transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neurol. 56, 965–973 (1997).
Frautschy, S. A. et al. Microglial response to amyloid plaques in APPSW transgenic mice. Am. J. Pathol. 152, 307 –317 (1998).
Bliss, T. V. P. & Collingridge, G. L. A synaptic model of memory—long-term potentiation in the hippocampus. Nature 361, 31–39 ( 1993).
Errington, M. L., Bliss, T. V. P., Morris, R. J., Laroche, S. & Davis, S. Long-term potentiation in awake mutant mice. Nature 387, 666–667 (1997).
Zucker, R. S. Short-term synaptic plasticity. Annu. Rev. Neurosci. 12, 13–31 (1989).
Hyman, B. T., Van Hoesen, G. W., Kromer, L. J. & Damasio, A. R. Perforant pathway change and the memory impairment of Alzheimer's disease. Ann. Neurol. 20, 473–482 (1986).
Finch, C. E. Neuron atrophy during aging: programmed or sporadic? Trends Neurosci. 16, 104–110 ( 1993).
Schauwecker, P. E. & Steward, O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc. Natl. Acad. Sci. USA 94, 4103–4108 (1997).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ 1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 95, 6448– 6453 (1998).
Nalbantoglu, J. et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387, 500–505 (1997).
Südhof, T. C. The synaptic vesicle cycle: a cascade of protein–protein interaction. Nature 375, 645–653 (1995).
Luthi, A., Laurent, J. P., Figurov, A., Muller, D. & Schachner, M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature 372, 777–779 (1994).
Dragunow, M. A role for immediate-early transcription factors in learning and memory. Behav. Genet. 26, 293–299 (1996).
Ikezu, T. et al. Negative transactivation of cAMP response element by familial Alzheimer's mutants of APP. EMBO J. 15, 2468–2475 (1996).
Castillo, P. E. et al. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388, 590– 593 (1997).
Kang, H., Welcher, A. A., Shelton, D. & Schuman, E. M. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 19, 653– 664 (1997).
Giese, K. P., Federov, N. B., Filipkowski, R. K. & Silva, A. J. Autophosphorylation at threonine 286 of the α calcium-calmodulin-kinase II in LTP and learning. Science 279, 870 (1998).
Klann, E., Chen, S. J. & Sweatt, J. D. Persistent protein kinase activation in the maintenance phase of long-term potentiation. J. Biol. Chem. 266 , 24253–24256 (1991).
Winder, D. G., Mansuy, I. M., Osman, M., Moallem, T. M. & Kandel, E. R. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell 92, 25–37 ( 1998).
Acknowledgements
The authors acknowledge the NIH (NS33249 (KH), K08-AG00793 (MI and BH), and AG08487 (BH)), the Mayo Medical Foundation (KH, SY) and the Medical Research Council (PC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chapman, P., White, G., Jones, M. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2, 271–276 (1999). https://doi.org/10.1038/6374
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/6374
This article is cited by
-
Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer’s Disease
Molecular Psychiatry (2024)
-
Role of sleep in neurodegeneration: the consensus report of the 5th Think Tank World Sleep Forum
Neurological Sciences (2024)
-
Critical thinking of Alzheimer’s transgenic mouse model: current research and future perspective
Science China Life Sciences (2023)
-
Differential vulnerability of hippocampal CA3-CA1 synapses to Aβ
Acta Neuropathologica Communications (2022)
-
Plasticity in visual cortex is disrupted in a mouse model of tauopathy
Communications Biology (2022)