Abstract
In 1998, we proposed deep brain stimulation as a last-resort treatment option for patients suffering from severe, treatment-resistant obsessive-compulsive disorder (OCD). Here, 24 OCD patients were included in a long-term follow-up study to evaluate the effects of electrical stimulation in the anterior limbs of the internal capsule (ALIC) and bed nucleus of the stria terminalis (BST). We find that electrical stimulation in the ALIC/BST area is safe and significantly decreases obsessions, compulsions, and associated anxiety and depressive symptoms, and improves global functioning in a blinded crossover trial (n=17), after 4 years (n=18), and at last follow-up (up to 171 months, n=24). Moreover, our data indicate that BST may be a better stimulation target compared with ALIC to alleviate OCD symptoms. We conclude that electrical stimulation in BST is a promising therapeutic option for otherwise treatment-resistant OCD patients.
Similar content being viewed by others
Introduction
Obsessive-compulsive disorder (OCD) is a highly disabling neuropsychiatric disorder, characterized by persistent, often anxiety-provoking thoughts (obsessions) and repetitive rituals (compulsions).1 It affects 2% of the population, and although pharmacological or cognitive behavioral therapy may reduce symptoms for most patients, about 10% remains severely incapacitated.2, 3
In 1998, we proposed deep brain stimulation (DBS) as a ‘last-resort’ treatment option for patients suffering from severe, treatment-refractory OCD.4 Compared with other neurosurgical interventions, for example, anterior capsulotomy, DBS has the advantage of being reversible and adjustable. Originally, stimulation targets were identical to those used in stereotactic ablative surgery (i.e. anterior limbs of the internal capsule (ALIC) for OCD), by analogy with DBS target selection in Parkinson’s disease.5 ALIC connects the frontal lobe and thalamus, as part of the cortico-striato-thalamo-cortical circuitry.6 This circuitry has been the prevailing neuroanatomical model of OCD pathophysiology during the past decades. Although valuable, recent evidence indicates that this model may still be refined and extended, especially by including brain regions implicated in (the expression of) anxiety.7 Following the first trials with DBS in ALIC,4, 8 additional cortico-striato-thalamo-cortical targets (e.g. nucleus accumbens) have been proposed.9 In the present study, clinical observations in the first patients10 and the intention to provide the best possible treatment made us shift from ALIC toward the bed nucleus of the stria terminalis (BST). Furthermore, several findings support the involvement of BST in anxiety, stress and compulsive behavior.11, 12, 13, 14
DBS for treatment-refractory OCD is an emerging opportunity, which should be seized with caution and under strict ethical evaluation.15 Since our first report on the initial effects in four patients, other research groups have followed (studies with ⩾4 patients8, 9, 10, 16, 17, 18, 19, 20), and over the years, we have published provisional data from some of our patients (e.g. Gabriëls et al.21 and Nuttin et al.,22 see Supplementary Appendix for details). Here, we report on the, to our knowledge, largest double-blind crossover study and longest open-label follow-up in OCD patients treated with DBS. In addition, we examine the relationship between psychiatric outcome and neuroanatomical location of the stimulation contacts.
Materials and methods
For detailed Materials and Methods, refer to Supplementary Appendix.
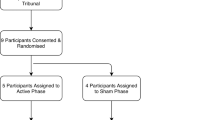
Twenty-four, stringently selected,23 severely affected OCD patients entered this study between 1998 and 2010. Included subjects suffered from severe to extreme OCD (Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) ⩾30) and had been seriously impaired in daily functioning (global assessment of functioning (GAF) ⩽45) for at least 5 years, despite extensive pharmacological and cognitive behavioral trials. The study protocol was reviewed and approved by the Ethics Committees of the University Hospitals of Leuven and Antwerp, and all patients provided their informed consent.
Quadripolar electrodes were stereotactically implanted into the bilateral ALIC, similar to the targets used for anterior capsulotomy. The most ventral contact (contact 0) was implanted in the gray matter ventral to ALIC and the other contacts (1–2–3) were placed in ALIC (lead tip position was 15 mm rostral to the posterior border of the anterior commissure in the first patient, and progressively more posterior toward the posterior border of the anterior commissure in later patients).10 Preliminary analyses correlating electrode position and Y-BOCS scores indicated a better outcome with a slightly more posterior target. Therefore, subsequent patients were implanted at this 'optimized' target, which was more posterior, ventral and medial, with at least one contact (usually contact 0) in the BST (0–2 mm posterior to the posterior border of the anterior commissure).
During the next months, stimulation parameters were optimized for each patient, the primary aim being a stable reduction in obsessive and compulsive symptoms. A 35% decrease in Y-BOCS score was defined as the responder criterion. Selection of parameters was based on immediate responses to DBS, for example, improvements in mood, anxiety and obsessions, without persisting side effects.24
After the initial parameter optimization phase, patients entered a double-blind, randomized (www.random.org) crossover study with two arms (3 months each, which could be truncated by the blinded psychiatrist if deemed necessary because of intolerable suffering), during which the patient was either stimulated (ON phase) or not (OFF phase). Medication was kept constant during the entire crossover study, and stimulation parameters remained unchanged throughout the ON phase. After completing both crossover arms (ON–OFF or OFF–ON), the patient and psychiatrist were unblinded, and the patient could choose to be continuously stimulated. The first patients have now been followed up for 16 years, whereas the last patients have had a follow-up of 4 years. In this paper, we will report on the stimulation effects for all patients, during the blinded crossover, 4 years after implantation and at last follow-up.
At several time points, patients were evaluated using standardized psychiatric questionnaires (primary outcome: Y-BOCS; secondary outcomes: Hamilton Anxiety and Depression Rating Scales (HAM-A and HAM-D) and GAF) and neuropsychological tests. In addition, we determined the position of the active electrode cathodes, while being blinded to the individual psychiatric outcome, to gain more insight into the exact locus of action. Finally, we analyzed if and how the patients’ outcome was related to the stimulated brain area.
Results
Patients
Twenty-four patients (12 male; Table 1) were selected according to strict selection criteria. Median age at implantation was 39 years. Median Y-BOCS was 35/40, indicating extreme OCD, and median GAF was 35/100, indicating major impairment in several areas of functioning. Seventeen patients (71%) completed the double-blind crossover trial (9 patients ON–OFF and 8 patients OFF–ON). Eighteen patients were followed up for 4 years or longer. The remaining six patients had a shorter follow-up period, because of cessation of stimulation within 4 years after implantation (2 patients), removal of the electrodes followed by capsulotomy (3 patients) or implantation of additional electrodes aimed at the subthalamic nucleus (1 patient). In addition, one patient who received stimulation for >10 years was not being stimulated at the moment of writing.
Adverse events
A total of 25 serious adverse events was recorded during the 180 patient-years of follow-up (Supplementary Table S1). Two out of 24 patients had an intracerebral hemorrhage during implantation of the electrodes. Other serious adverse events include four suicide attempts (three patients) and eight fractures and polytraumas due to accidents (eight patients). Five patients developed epileptic seizures: tonic–clonic insults (two patients), absences or partial seizures (three patients), without quantifiable neurological or cardiovascular abnormalities, as assessed with interictal electroencephalogram and Holter monitoring. Two patients were diagnosed with severe obstructive apnea. Four patients experienced other serious physical events: deterioration of obesity to morbid obesity, pyelonephritis, transient ischemic attack and early prostate carcinoma. For an overview of all device-related and other adverse events (Supplementary Table S2)—none of which were life threatening or resulted in any permanent injury, see Supplementary Appendix.
Crossover trial
Stimulation parameters
Stimulation parameters and electrode polarity (Supplementary Table S3) were optimized for each individual patient based on clinical evaluation. Stimulation frequencies ranged from 85 to 130 Hz (median 130 Hz), pulse widths from 90 to 450 μs (median 240 μs) and amplitudes from 3 to 10.5 V (median 6.5 V). The average (±s.d.) charge density, estimated with a 1 kOhm impedance, was 19.9 (±12.5) μC cm−2, which is in line with previously reported charge densities.25
Target
To determine the exact location of the active contacts, postoperative magnetic resonance scans were used (10 patients). For three other patients, preoperative magnetic resonance and postoperative computed tomography scans were merged. For the four earliest patients, no digital images were available; therefore, the available scans (magnetic resonance and/or computed tomography) were examined visually, without further merging or processing.
Five patients had bilateral contacts in BST (Figure 1), and another patient was stimulated unilaterally in BST. Two patients were stimulated in BST in one hemisphere and in IC, adjacent to BST, in the other hemisphere. Two patients received bilateral stimulation in IC or the prereticular zone, adjacent to BST. Five patients received bilateral ALIC stimulation. Two patients were stimulated in BST and ALIC.
Coronal brain slices showing the location of the center of all active electrode cathodes, as well as the stimulated area, during the crossover ON phase (17 patients). Contacts are depicted with different symbols, according to the patient’s Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) improvement during the ON phase (vs OFF): ●, <25% improvement; ▴, 25–35%; ▪, 35–65% and ★, >65% improvement. For each patient, the stimulated volume was calculated (see Supplementary Table S3) and indicated by the colored radii extending from the contacts (the radius is only shown on the slice containing the center of the contact, although it may extend over adjacent slices). Note that some patients were stimulated with more than one contact, for example, the three contacts shown on the slice at −15.0 mm are from one patient, as indicated by the extent and shape of the stimulated volume. In the top left corner of each slice, the position anterior (−) or posterior (+) to the anterior commissure is specified. Instead of showing complete coronal slices, a detailed window is shown, dorsally bordered by the corpus callosum and ventrally extending 10 mm below the intercommissural plane, as indicated in the bottom left panel. Additionally, a sagittal view of the brain with indication of the relevant coronal slices is shown. 3 V, third ventricle; ac, anterior commissure; Ac, nucleus accumbens; ALIC, anterior limb of the internal capsule; BST, bed nucleus of the stria terminalis; cc, corpus callosum; Cd, caudate nucleus; DPeH, dorsal periventricular hypothalamic nucleus; EGP, external globus pallidus; FCd, fundus region of caudate nucleus; FLV, frontal horn of lateral ventricle; FPu, fundus region of putamen; fx, fornix; gcc, genu of the corpus callosum; GTI, great terminal island; IC, internal capsule; LH, lateral hypothalamic area; lml, lateral medullary lamina of globus pallidus; LS, lateral septal nucleus; LV, lateral ventricle; mml, medial medullary lamina of globus pallidus; MPO, medial preoptic nucleus; OlfA, olfactory area; Pa, paraventricular nucleus; PRt, prereticular zone; Pu, putamen; SCGP, supracapsular part of globus pallidus; st, stria terminalis. Images are adapted from Mai’s Atlas of the Human Brain.41
Taking into consideration the stimulated volume radiating from the active contacts (Figure 1), we found that most patients (82%) were stimulated in BST, and/or in IC (41%) or its anterior limb (35%). Each patient received stimulation in at least one of these three areas. Because of its proximity to BST, several patients (47%) were stimulated in or bordering the prereticular zone. Other structures that were often partially included in the stimulation radius were the external globus pallidus (59%) and parts of the lateral septal nucleus (53%). Note that only two patients (12%) received (partial) stimulation of the nucleus accumbens. Besides (AL)IC, other white matter structures, such as the anterior commissure and fornix were frequently (41%) located in the stimulated area. Other structures close to BST and (AL)IC, which were often in the periphery of the stimulation range, were the lateral or medial medullary lamina of the globus pallidus (53%), caudate nucleus and putamen (41%) and paraventricular nucleus (35%).
For the 17 crossover patients, cathodes were largely the same during crossover, optimal open-label period and at last follow-up, resulting in comparable stimulated areas at these three time points (for details, see Supplementary Appendix).
Psychiatric outcome
Patients showed a significant improvement (median: 37%) in Y-BOCS scores when comparing the blinded ON phase (median Y-BOCS score: 20) with the blinded OFF phase (median Y-BOCS score: 32) (Figure 2a and Table 2). This suggests that the beneficial effect is indeed caused by electrical stimulation and is not a mere (placebo) effect of implantation. Note that 13 out of 17 patients also had improved Y-BOCS scores (median improvement: 11%) during the OFF phase as compared with the preoperative situation (median Y-BOCS score: 35), which might indicate some placebo effect or potentially a slight microlesion effect because of the insertion of electrodes. Finally, Y-BOCS scores were significantly better during the blinded ON phase as compared with the preoperative situation (median improvement: 42%).
Primary (Y-BOCS) and secondary (HAM-A, HAM-D, GAF) psychiatric outcome measures. Individual test data are shown for the crossover trial (n=17), and for the comparison between the preoperative situation and after 4 years of follow-up (n=18), and at last follow-up (n=24) (a–l). Black arrows in graphs (c), (f), (i) and (l) indicate patients whose last follow-up measurement was taken before capsulotomy (n=3), cessation of stimulation (n=2) or implantation of additional electrodes in the subthalamic nucleus (n=1). These six patients have a follow-up duration of <4 years, and are thus not included in graphs (b), (e), (h) and (k). The dotted arrows in graphs (c), (f), (i) and (l) indicate a patient who is currently not being stimulated, but received stimulation for 10 years, and is thus also included in graphs (b), (e), (h) and (k). Statistically significant differences, as calculated with Wilcoxon's matched-pairs tests (see Table 3 for details), are indicated: *P<0.017, **P<0.001 and ***P<0.0001. 4YFU, 4-year follow-up; GAF, global assessment of functioning; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale; LFU, last follow-up; preop, preoperative; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale.
We found similar improvements in HAM-A, HAM-D and GAF scores (Figures 2d, g and j and Table 2): significant improvement during ON vs OFF (median improvement: 67%, 58% and 15 points, respectively), and vs the preoperative situation (median improvement: 71%, 54% and 30 points, respectively). For HAM-A and HAM-D scores, we found no significant 'placebo effect', that is, these scores were not better during OFF as compared with the preoperative status. GAF scores, on the other hand, were slightly better during OFF as compared with the preoperative status (median improvement: 5 points).
Although both crossover phases were intended to have a duration of 3 months, the ON phase was shortened for 3 out of 17 patients (even though two of these patients objectively improved, with a reduction of ⩾9 Y-BOCS points). Fourteen patients used the escape procedure during the OFF phase, because of unbearable worsening of the symptoms. The median duration of the ON phase (89 days) was significantly longer than the OFF phase (44 days) (Wilcoxon's matched-pairs test, Z=3.05, P<0.01).
Taken together, these analyses provide strong evidence for beneficial effects of electrical stimulation on obsessions and compulsions (Y-BOCS), anxiety and depressive symptoms (HAM-A, HAM-D) and improvement of functioning (GAF) in these otherwise treatment-resistant OCD patients.
Neuropsychological and qualitative analyses
Neuropsychological evaluations were performed before surgery and during both crossover phases. For a complete overview of all (sub)test scores and statistical analyses, see Supplementary Appendix and Supplementary Table S4. Our data indicate that DBS lowered scores on subtests of the Stroop test (suggesting less interference and improved executive functioning) and Trail Making Test (indicating better performance on this assessment of visual search, scanning, speed of processing, mental flexibility and executive functions). DBS also increased scores on subtests of the Auditory Verbal Learning Test (supporting improved verbal learning and memory). In conclusion, the neuropsychological tests indicated no stimulation-induced cognitive or memory impairment.
A qualitative analysis (Supplementary Appendix) of the available descriptive medical reports pointed out several potential side effects (poor sleep quality, weight changes, libido changes, urinary incontinence and physical inconveniences inherent to DBS, for example, sensation of the stimulator in the abdomen) that might be examined in more detail in future studies and monitored in clinical practice.
Follow-up
Target
Seven patients did not enter the crossover part of the study, because of several reasons. Five of them had very good effects and/or did not want to relive the 'stimulation off' situation, which they had already encountered with empty batteries. One patient experienced only slight improvement (lowest Y-BOCS score was 26/40) and underwent capsulotomy after 28 months. One patient, who was rather capricious, refused to start the crossover design and is currently not being stimulated, although the objective effects of DBS were good (lowest Y-BOCS was 12 and at last follow-up it was 15, indicating mild OCD).
The active stimulation cathodes for the seven 'non-crossover' patients were mainly located in BST (bilaterally in four patients, left side only in one other patient, with the right contact in IC and external globus pallidus, adjacent to BST). Two patients had bilateral cathodes in ALIC and/or external globus pallidus, adjacent to BST (one patient) or nucleus accumbens (one patient).
For these seven patients, negative contacts during the optimal open-label period (⩾2 months) remained unaltered at last follow-up, apart from one patient who had an additional cathode on the left side at last follow-up, further elongating the stimulated volume into more ventral portions of BST (Supplementary Appendix).
Psychiatric outcome
Four years after implantation, there was a significant improvement in Y-BOCS, HAM-A, HAM-D and GAF scores, as compared with the preoperative situation (Figures 2b, e, h and k and Table 2). The median improvements in test scores were 66%, 58%, 67% and 30 points, respectively. Note that the Y-BOCS score after 4 years was highly correlated with the ON score during the crossover trial (Spearman's R=0.83, P<0.001).
For 3 out of 24 patients, last follow-up took place at 4 years. For six patients, last follow-up data were obtained before 4 years postimplantation, because of reasons listed above. For the remaining 15 patients, last follow-up scores were recorded after >4 years postimplantation (range: 54–171 months).
At last follow-up, there was a significant improvement in Y-BOCS, HAM-A, HAM-D and GAF scores, as compared with the preoperative situation (Figures 2c, f, i and l and Table 2). The median improvements in test scores were 45%, 45%, 49% and 30 points, respectively. Sixteen out of 24 patients (67%) reached the responder criterion. Note that the Y-BOCS score at last follow-up was highly correlated with the ON score during the crossover trial (Spearman's R=0.83, P<0.001).
In conclusion, we found that electrical stimulation in the ALIC/BST area significantly decreases obsessions and compulsions (Y-BOCS), associated anxiety and depression (HAM-A, HAM-D) and improves functioning (GAF) in our OCD patients, not only during a double-blind, randomized crossover trial but also after 4 years of follow-up (18 patients), and at last follow-up (24 patients).
Target vs outcome
As mentioned above, each patient was stimulated in BST, IC adjacent to BST and/or in ALIC. To investigate if one of both main targets (i.e., BST or ALIC) resulted in a better outcome compared with the other at last follow-up, we subdivided all 24 patients into three groups: those stimulated primarily in ALIC (6 patients), primarily in BST (15 patients) or unclassifiable patients with comparable stimulation of ALIC and BST (3 patients) (Table 3). Using the predefined criterion of at least 35% Y-BOCS improvement to define a satisfactory effect of stimulation, we analyzed the stimulation target vs the long-term follow-up outcome in a 2 × 2 contingency table. Five ALIC-stimulated patients showed insufficient effects at last follow-up, whereas one ALIC patient showed a favorable effect. Three BST-stimulated patients showed insufficient effects, whereas 12 patients showed a satisfactory effect. A Fisher's exact test (P=0.01) indicated that BST stimulation had significantly better effects compared with ALIC stimulation, which was also reflected by the average Y-BOCS improvement at last follow-up: 22% for ALIC stimulation and 50% for BST stimulation. Similar effects were found when analyzing Y-BOCS improvement during the crossover phase, although this finding could not be substantiated statistically. Better therapeutic effects with BST stimulation could not be explained by higher stimulation parameters. At last follow-up, stimulation voltage (mean±s.d.) for both targets was comparable (BST: 6.6±2.1 V; ALIC: 6.7±2.3 V), along with charge density (BST: 20.3±11.4 μC cm−2; ALIC: 22.1±10.5 μC cm−2) and frequency (130 Hz for all patients, except for two ALIC patients—one responder and one non-responder—who were stimulated at 100 Hz). A Mann–Whitney U-test did indicate longer pulse widths (median (range)) in ALIC patients (BST: 210 μs (120–450 μs); ALIC: 345 μs (240–450 μs)), reflecting attempts to optimize therapeutic effects for these patients. Note that the three 'unclassifiable' patients with commensurable stimulation of ALIC and BST all experienced very good effects at last follow-up (66% Y-BOCS improvement on average). Finally, we would like to point out that 50% (3/6) of the patients primarily stimulated in ALIC are currently not being stimulated (capsulotomy or stimulation turned off), whereas this is 20% (3/15) in the BST-stimulated patients.
To conclude, our data suggest that BST might be a better stimulation target than ALIC to reduce obsessions and compulsions in OCD patients.
Discussion
In this long-term clinical study, we found that electrical stimulation in the ALIC/BST region substantially alleviates OCD symptoms in otherwise treatment-refractory patients. Therapeutic effects were corroborated in a double-blind, randomized crossover phase, after 4 years and at last follow-up. Electrical stimulation also reduced associated anxiety and depressive symptoms, and improved overall functioning. Additionally, our data indicate that BST may be a more effective stimulation target compared with ALIC.
Although promising as a last-resort treatment option for OCD, DBS should be applied with care, given the inherent risks of a surgical procedure (e.g. intracerebral hemorrhage). Notwithstanding our encouraging results, this treatment remains experimental, and should be scrutinized in carefully controlled clinical trials, before being offered as a standard treatment option.15, 23
An observation that deserves particular attention is the development of epileptic seizures in five patients. Although never confirmed by interictal electroencephalogram abnormalities and perhaps unrelated to DBS, this should be taken seriously. Patient 19 cited seizures as one of his motives to discontinue stimulation, together with insufficient therapeutic effects. In the literature, we found one instance of seizures in OCD patients, that is, a single tonic–clonic insult in the surgery room after lead implantation in ALIC.26 Other research groups might not have encountered postoperative seizures because of smaller sample sizes and shorter follow-up or because of different stimulation targets. In our study, seizures emerged after 2–5 years of bilateral BST stimulation. Evidence directly implicating BST in epilepsy is scarce, and we can only speculate on their association. However, it is noteworthy that BST projects to the paraventricular nucleus of the hypothalamus, a key element of the hypothalamic-pituitary-adrenal axis, and stress is most likely an important trigger for seizures.27, 28 In addition, there are indications for partially overlapping neural circuitries of OCD and epilepsy.29 On a side note, (predominantly thalamic) DBS has been used for decades to treat epilepsy.30
Some of our patients had mild memory complaints, similar to subjective reports in other DBS for OCD studies.16 Interestingly, double-blind evaluation of neuropsychological functioning during the crossover phases provided no evidence for stimulation-induced cognitive impairment, but rather the opposite.
Blinded crossover studies are of paramount importance in this evolving field, to thoroughly assess safety and efficacy of DBS in OCD. We recognize that partial unblinding may have occurred in our trial, as stimulation may have certain side effects (e.g. paresthesias) that could make the patient aware of the stimulation condition. We opted for the use of optimized parameters instead of using settings below the side-effect threshold, which may be insufficient to achieve therapeutic effects.17 Note that assumptions about the stimulation state were never confirmed by the (unblinded) neurosurgeon.
One of our main findings is the effectiveness of BST as a stimulation target in OCD. Although BST is not part of the classical cortico-striato-thalamo-cortical circuitry, it has been implicated in anxiety, stress and compulsions. More specifically, there is evidence for a role of BST in sustained expression of anxiety, for example, in context conditioning paradigms.12, 13, 31 Also, it appears to be involved in anticipatory anxiety and threat monitoring.32, 33, 34 Moreover, BST may mediate the stress response through its connections with the hypothalamus and its large amount of corticotropin-releasing factor responsive neurons.35 The present study provides the first clinical evidence for BST involvement in OCD, which is supported by preclinical findings.14, 36 To better grasp the mechanisms of BST stimulation in OCD, it may be worthwhile to monitor closely the sequence of therapeutic effects in future studies. We and others have previously described that effects on mood and anxiety are usually noted first, before apparent changes in obsessions or compulsions.10, 16, 37 Given the key role of BST in anxiety, it may be interesting to examine if initial anxiolytic effects drive the attenuation of OCD symptoms.
It has been argued that the classical neurocircuitry model of OCD should be extended to include other brain areas, for example, those implicated in the expression of fear and anxiety.7 Based on prior and present findings, BST may be a good candidate. This supposition is further supported by nuclear imaging data from 16 of our patients.38, 39 Chronic stimulation in the ALIC/BST area induced changes in components of the cortico-striato-thalamo-cortical loops, that is, metabolic decreases in the anterior cingulate cortex (which was elevated preoperatively as compared with healthy subjects) and in the prefrontal and orbitofrontal cortices.
Previous crossover studies used 35% Y-BOCS reduction after surgery as the responder criterion (our comparison of ON vs OFF was more stringent and controlled for placebo effects) or did not define responder criteria, but reported the percentage Y-BOCS improvement instead. They found response rates of 10–25%, or average improvements of 25–31%.8, 16, 17, 40 Open-label studies (3–36 months follow-up) reported response rates of 10–56%, up till 100% (only in small trials, n=4–6).8, 10, 16, 18, 19, 40
In this study, we found 53% responders (median improvement 63%) during the crossover trial and 67% responders at last follow-up (median improvement 58%). When restricting this analysis to patients who received stimulation in the presumably better target, that is, BST (n=18), we found 67% responders during the crossover trial and 83% responders at last follow-up.
It remains unclear why DBS in BST (and ALIC) seems to have extraordinary results in some patients, yet unsatisfactory effects in others. Fortunately, its reversible nature allows for other interventions, such as capsulotomy, thereby giving these patients another chance of successful treatment, as was the case in two out of three patients who underwent subsequent capsulotomy in this study. Continued research in larger patient samples is needed to shed light on potential differences in the eligibility of patients with different OCD subtypes or comorbidities. Furthermore, fundamental research clarifying the mechanisms of stimulation in specific targets may be instrumental in refining DBS as a treatment for psychiatric disorders.
References
American Psychiatric Association Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th (edn). American Psychiatric Association: Washington, DC, 2000.
Denys D . Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin N Am 2006; 29: 553–584.
Jenike MA . Clinical practice. Obsessive-compulsive disorder. N Engl J Med 2004; 350: 259–265.
Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B . Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet 1999; 354: 1526.
Benabid AL . Deep brain stimulation for Parkinson's disease. Curr Opin Neurobiol 2003; 13: 696–706.
Lopes AC, Greenberg BD, Noren G, Canteras MM, Busatto GF, de Mathis ME et al. Treatment of resistant obsessive-compulsive disorder with ventral capsular/ventral striatal gamma capsulotomy: a pilot prospective study. J Neuropsychiatry Clin Neurosci 2009; 21: 381–392.
Milad MR, Rauch SL . Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012; 16: 43–51.
Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 2005; 57: 510–516.
Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat 2003; 26: 293–299.
Greenberg BD, Gabriëls LA, Malone DA Jr, Rezai AR, Friehs GM, Okun MS et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 2010; 15: 64–79.
Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP . Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 2007; 27: 2025–2034.
Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 2010; 35: 105–135.
Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B . Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav Brain Res 2011; 222: 357–362.
van Kuyck K, Brak K, Das J, Rizopoulos D, Nuttin B . Comparative study of the effects of electrical stimulation in the nucleus accumbens, the mediodorsal thalamic nucleus and the bed nucleus of the stria terminalis in rats with schedule-induced polydipsia. Brain Res 2008; 1201: 93–99.
Fins JJ, Mayberg HS, Nuttin B, Kubu CS, Galert T, Sturm V et al. Misuse of the FDA's humanitarian device exemption in deep brain stimulation for obsessive-compulsive disorder. Health Aff 2011; 30: 302–311.
Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2010; 67: 1061–1068.
Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 2008; 359: 2121–2134.
Jiménez F, Nicolini H, Lozano AM, Piedimonte F, Salin R, Velasco F . Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg 2013; 80: e17–e25.
Roh D, Chang WS, Chang JW, Kim CH . Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res 2012; 200: 1067–1070.
Tsai HC, Chang CH, Pan JI, Hsieh HJ, Tsai ST, Hung HY et al. Pilot study of deep brain stimulation in refractory obsessive-compulsive disorder ethnic Chinese patients. Psychiatry Clin Neurosci 2012; 66: 303–312.
Gabriëls L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J . Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand 2003; 107: 275–282.
Nuttin B, Gabriëls L, Cosyns PR, Meyerson BA, Andréewitch S, Sunaert SG et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 2003; 52: 1263–1272.
Nuttin B, Wu H, Mayberg H, Hariz M, Gabriëls L, Galert T et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry 2014; 85: 1003–1008.
Nuttin B, Gabriëls L, van Kuyck K, Cosyns P . Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am 2003; 14: 267–274.
Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS . Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PLoS One 2013; 8: e58665.
Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006; 31: 2384–2393.
Heinrichs SC . Neurobehavioral consequences of stressor exposure in rodent models of epilepsy. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 808–815.
Maguire J, Salpekar JA . Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav 2013; 26: 352–362.
Kaplan PW . Obsessive-compulsive disorder in chronic epilepsy. Epilepsy Behav 2011; 22: 428–432.
Sprengers M, Vonck K, Carrette E, Marson AG, Boon P . Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 2014; 6: CD008497.
Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K et al. Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. J Neurosci 2012; 32: 254–263.
Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C . Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage 2011; 55: 389–400.
Somerville LH, Whalen PJ, Kelley WM . Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 2010; 68: 416–424.
Straube T, Mentzel HJ, Miltner WH . Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. NeuroImage 2007; 37: 1427–1436.
Walker DL, Toufexis DJ, Davis M . Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 2003; 463: 199–216.
Wu H, Tambuyzer T, Nica I, van Kuyck K, Aerts J-M, Van Huffel S et al. Delta oscillations in the bed nucleus of the stria terminalis correlate with compulsion in a rat model of obsessive-compulsive disorder. (submitted).
Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR et al. What's in a 'smile?' Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase 2004; 10: 271–279.
Suetens K, Nuttin B, Gabriels L, Van Laere K . Differences in metabolic network modulation between capsulotomy and deep-brain stimulation for refractory obsessive-compulsive disorder. J Nucl Med 2014; 55: 951–959.
Van Laere K, Nuttin B, Gabriëls L, Dupont P, Rasmussen S, Greenberg BD et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med 2006; 47: 740–747.
Huff W, Lenartz D, Schormann M, Lee SH, Kuhn J, Koulousakis A et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg 2010; 112: 137–143.
Mai JK, Paxinos G, Voss T . Atlas of the Human Brain, 3rd (edn). Elsevier/Academic Press: London, UK, 2008.
Acknowledgements
This work was supported by the Research Foundation—Flanders (FWO) Project G072909N, FWO Research Grant (to LL) 1504614N, KU Leuven Grant PF/10/005 and by the Agency for Innovation by Science and Technology (IWT-SBO090054). LL is a postdoctoral fellow of the FWO. All devices were generously provided by Medtronic. They also provided grants for research, education and traveling (to BN and LG), who hold the Medtronic Chair for Stereotactic Neurosurgery in Psychiatric Disorders at KU Leuven. BN co-owns a patent on DBS in OCD. We are grateful to our colleagues at the lab of Experimental Neurosurgery who have conducted parallel preclinical research for years, thereby laying the foundation for and scrutinizing the clinical findings. We thank Prof. Dr Jürgen K Mai and Dr Milan Majtanik for providing high-resolution images of the human brain atlas. We also thank the members of the Committee for Neurosurgery for Psychiatric Disorders, and the hospital staff and John Das for taking excellent care of the patients. Finally, we would like to thank all participating patients and their families.
Author contributions
LL conducted all analyses and wrote the article. BN initiated the clinical trial, performed all surgeries, followed up all patients, and revised the manuscript. LG psychiatrically evaluated and followed up all patients, and revised the manuscript. SH contributed to the data collection for the qualitative analysis of the crossover trial and SR to the data collection for the analysis of the open-label adverse events. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Luyten, L., Hendrickx, S., Raymaekers, S. et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry 21, 1272–1280 (2016). https://doi.org/10.1038/mp.2015.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.124
This article is cited by
-
Closing the loop in psychiatric deep brain stimulation: physiology, psychometrics, and plasticity
Neuropsychopharmacology (2024)
-
Gamma knife capsulotomy for intractable OCD: Neuroimage analysis of lesion size, location, and clinical response
Translational Psychiatry (2023)
-
The prefrontal cortex and neurosurgical treatment for intractable OCD
Neuropsychopharmacology (2022)
-
Patient-specific connectomic models correlate with, but do not reliably predict, outcomes in deep brain stimulation for obsessive-compulsive disorder
Neuropsychopharmacology (2022)
-
Comment to: Deep brain stimulation for refractory obsessive-compulsive disorder (OCD): emerging or established therapy?
Molecular Psychiatry (2022)