Abstract
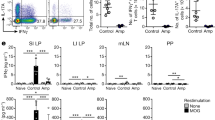
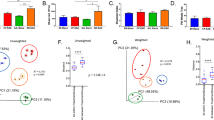
Active multiple sclerosis lesions show inflammatory changes suggestive of a combined attack by autoreactive T and B lymphocytes against brain white matter1. These pathogenic immune cells derive from progenitors that are normal, innocuous components of the healthy immune repertoire but become autoaggressive upon pathological activation. The stimuli triggering this autoimmune conversion have been commonly attributed to environmental factors, in particular microbial infection2. However, using the relapsing–remitting mouse model of spontaneously developing experimental autoimmune encephalomyelitis3, here we show that the commensal gut flora—in the absence of pathogenic agents—is essential in triggering immune processes, leading to a relapsing–remitting autoimmune disease driven by myelin-specific CD4+ T cells. We show further that recruitment and activation of autoantibody-producing B cells from the endogenous immune repertoire depends on availability of the target autoantigen, myelin oligodendrocyte glycoprotein (MOG), and commensal microbiota. Our observations identify a sequence of events triggering organ-specific autoimmune disease and these processes may offer novel therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Franciotta, D. et al. B cells and multiple sclerosis. Lancet Neurol. 79, 852–858 (2008)
Ascherio, A. & Munger, K. L. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 61, 288–299 (2007)
Pöllinger, B. et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med. 206, 1303–1316 (2009)
Goverman, J. et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72, 551–560 (1993)
Hill, D. A. & Artis, D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 28, 623–667 (2010)
Lampropoulou, V. et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J. Immunol. 180, 4763–4773 (2008)
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 108, 4615–4622 (2011)
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008)
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009)
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009)
Hall, J. A. et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 (2008)
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011)
Ercolini, A. M. & Miller, S. D. Molecular mimics can induce novel self peptide-reactive CD4+ T cell clonotypes in autoimmune disease. J. Immunol. 179, 6604–6612 (2007)
Feng, T. et al. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 207, 1321–1332 (2010)
Kirvan, C. A., Cox, C. J., Swedo, S. E. & Cunningham, M. W. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J. Immunol. 178, 7412–7421 (2007)
Delarasse, C. et al. Myelin/oligodendrocyte glycoprotein-deficient (MOG-deficient) mice reveal lack of immune tolerance to MOG in wild-type mice. J. Clin. Invest. 112, 544–553 (2003)
Cyster, J. G. B cell follicles and antigen encounters of the third kind. Nature Immunol. 11, 989–996 (2010)
McMahon, E. J. et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature Med. 11, 335–339 (2005)
Cserr, H. F., Harling-Berg, C. J. & Knopf, P. M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2, 269–276 (1992)
Schwickert, T. A., Alabyev, B., Manser, T. & Nussenzweig, M. C. Germinal center reutilization by newly activated B cells. J. Exp. Med. 206, 2907–2914 (2009)
Litzenburger, T. et al. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J. Exp. Med. 188, 169–180 (1998)
Barnett, M. H., Henderson, A. P. D. & Prineas, J. W. The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Ms 12, 121–132 (2006)
Hohlfeld, R. Multiple sclerosis: human model for EAE? Eur. J. Immunol. 39, 2036–2039 (2009)
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010)
Vaahtovuo, J. et al. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 35, 1500–1505 (2008)
Knip, M. et al. Dietary intervention in infancy and later signs of β-cell autoimmunity. N. Engl. J. Med. 363, 1900–1908 (2010)
Lauer, K. Diet and multiple sclerosis. Neurol. (Tokyo) 49 (suppl. 2). S55–S61 (1997)
Kira, J.-I. et al. Changes in the clinical phenotypes of multiple sclerosis during the past 50 years in Japan. J. Neurol. Sci. 166, 53–57 (1999)
Acknowledgements
We thank I. Arnold-Ammer, N. Reißer and L. Penner for technical assistance, and M. Pfunder, U. Stauffer, C. Hornung and N. Joswig for maintaining our germ-free colony and technical support. We are much obliged to R. Kemler for his support. This work was funded by SFB 571 (Project B6), the German Competence Network on Multiple Sclerosis (KKNMS), ARSEP (France), and by the Max Planck Society. K.B. is supported by a fellowship from ARSEP. Z.A.R. is supported by a PhD fellowship from the Emirates Foundation. M.B. receives a fellowship from the Hellenic Neurological Society.
Author information
Authors and Affiliations
Contributions
K.B., H.W. and G.K. designed experiments and wrote the manuscript with input from co-authors. K.B. performed most of the experiments. M.M., M.K. and M.B. performed flow cytometry experiments or assisted in experiments. Z.A.R. performed flow cytometry and immunofluorescence staining for brain infiltrates. C.J. supervised the maintenance of germ-free mouse colony and colonization experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-8 with legends and Supplementary Table 1. (PDF 831 kb)
Rights and permissions
About this article
Cite this article
Berer, K., Mues, M., Koutrolos, M. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011). https://doi.org/10.1038/nature10554
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10554
This article is cited by
-
Gut Microbiota and Autoimmune Diseases: Mechanisms, Treatment, Challenges, and Future Recommendations
Current Clinical Microbiology Reports (2024)
-
The gut microbiome regulates astrocyte reaction to Aβ amyloidosis through microglial dependent and independent mechanisms
Molecular Neurodegeneration (2023)
-
The gut microbiome in Alzheimer’s disease: what we know and what remains to be explored
Molecular Neurodegeneration (2023)
-
Myelin in Alzheimer’s disease: culprit or bystander?
Acta Neuropathologica Communications (2023)
-
GPR43 stimulation on TCRαβ+ intraepithelial colonic lymphocytes inhibits the recruitment of encephalitogenic T-cells into the central nervous system and attenuates the development of autoimmunity
Journal of Neuroinflammation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.