Abstract
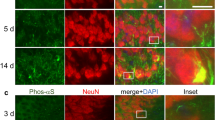
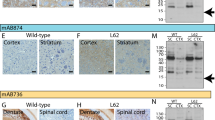
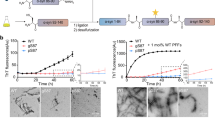
The deposition of the abundant presynaptic brain protein α-synuclein as fibrillary aggregates in neurons or glial cells is a hallmark lesion in a subset of neurodegenerative disorders. These disorders include Parkinson's disease (PD), dementia with Lewy bodies (DLB) and multiple system atrophy, collectively referred to as synucleinopathies1,2. Importantly, the identification of missense mutations in the α-synuclein gene in some pedigrees of familial PD has strongly implicated α-synuclein in the pathogenesis of PD and other synucleinopathies3. However, specific post-translational modifications that underlie the aggregation of α-synuclein in affected brains have not, as yet, been identified. Here, we show by mass spectrometry analysis and studies with an antibody that specifically recognizes phospho-Ser 129 of α-synuclein, that this residue is selectively and extensively phosphorylated in synucleinopathy lesions. Furthermore, phosphorylation of α-synuclein at Ser 129 promoted fibril formation in vitro. These results highlight the importance of phosphorylation of filamentous proteins in the pathogenesis of neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Spillantini, M. G. et al. Nature 388, 839–840 (1997).
Baba, M. et al. Am. J. Pathol. 152, 879–884 (1998).
Polymeropoulos, M. H. et al. Science 276, 2045–2047 (1997).
Jakes, R. et al. Neurosci. Lett. 269, 13–16 (1999).
Culvenor, J. G. et al. Am. J. Pathol. 155, 1173–1181 (1999).
Masliah, E. et al. Science 287, 1265–1269 (2000).
Sharon, R. et al. Proc. Natl Acad. Sci. USA 98, 9110–9115 (2001).
Okochi, M. et al. J. Biol. Chem. 275, 390–397 (2000).
Pronin, A. N., Morris, A. J., Surgunchov, A. & Benovic, J. L. J. Biol. Chem. 275, 26515–26522 (2000).
Lee, V. M.-Y., Balin, B. J., Otvos, L. Jr & Trojanowski, J. Q. Science 251, 675–678 (1991).
Goedert, M. Nature 399, 739–740 (1999).
Goldberg M. S. & Lansbury P. T. Jr Nature Cell Biol. 2, E115–E119 (2000).
Conway, K. A. et al. Proc. Natl Acad. Sci. USA 97, 571–576 (2000).
Giasson, B. I. et al. Science 290, 985–989 (2000).
Shimura, H. et al. Science 293, 263–269 (2001).
Hasegawa, M. et al. J. Biol. Chem. 267, 17047–17054 (1992).
Pandey, A. & Mann, M. Nature 405, 837–846 (2000).
Morishima-Kawashima, M. et al. J. Biol. Chem. 270, 823–829 (1995).
Tu, P.-H. et al. Ann. Neurol. 44, 415–422 (1998).
Iwatsubo, T., Hasegawa, M. & Ihara, Y. Acta Neuropathol. 88, 129–136 (1994).
Biere, A. L. et al. J. Biol. Chem. 275, 34574–34579 (2000).
Giasson, B. I., Murray, I. V., Trojanowski, J. Q. & Lee, V. M.-Y. J. Biol. Chem. 276, 2380–2386 (2001).
Crowther, R. A., Jakes, R., Spillantini, M. G. & Goedert, M. FEBS Lett. 436, 309–312 (1998).
Rochet, J.-C., Conway, K. A. & Lansbury, P. T. Jr Biochemistry 39, 10619–10626 (2000).
Volles, M. J. et al. Biochemistry 40, 7812–7819 (2001).¶
Acknowledgements
We thank P.T. Lansbury Jr. and J.-C. Rochet for advice on oligomer formation experiments, J.Q. Trojanowski, V.M.-Y. Lee and D.W. Dickson for frozen DLB samples, D.M.A. Mann for helpful comments on the manuscript and brain samples, M. Goedert for an expression plasmid encoding recombinant α-synuclein, Y. Ihara and T. Katada for making the TOF/MS and fluorospectrometer available, respectively, H. Nishina and D. Kitagawa for advice on in vitro phosphorylation experiments, T. Hashimoto for assistance in thioflavin-T and oligomer assays, M. Baba and T. Kuwahara for technical assistance, and A. Koyama, H. Miake and M. Takahashi for helpful discussions. This work was supported by grants-in-aid from the Ministry of Health and Welfare and the Ministry of Education, Science, Culture and Sports, Japan.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary figure
Figure S1 Quantitative analysis of the extent of α-synuclein phosphorylation in the urea-soluble fractions of DLB brains. (PDF 600 kb)
Rights and permissions
About this article
Cite this article
Fujiwara, H., Hasegawa, M., Dohmae, N. et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4, 160–164 (2002). https://doi.org/10.1038/ncb748
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb748
This article is cited by
-
The involvement of α-synucleinopathy in the disruption of microglial homeostasis contributes to the pathogenesis of Parkinson’s disease
Cell Communication and Signaling (2024)
-
Dopaminergic neuron loss in mice due to increased levels of wild-type human α-Synuclein only takes place under conditions of accelerated aging
Scientific Reports (2024)
-
HTRA1 disaggregates α-synuclein amyloid fibrils and converts them into non-toxic and seeding incompetent species
Nature Communications (2024)
-
An artificial protein modulator reprogramming neuronal protein functions
Nature Communications (2024)
-
Linking acetylated α-Tubulin redistribution to α-Synuclein pathology in brain of Parkinson’s disease patients
npj Parkinson's Disease (2024)