Abstract
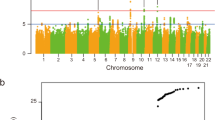
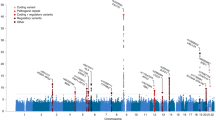
To identify susceptibility genes for amyotrophic lateral sclerosis (ALS), we conducted a genome-wide association study (GWAS) in 506 individuals with sporadic ALS and 1,859 controls of Han Chinese ancestry. Ninety top SNPs suggested by the current GWAS and 6 SNPs identified by previous GWAS were analyzed in an independent cohort of 706 individuals with ALS and 1,777 controls of Han Chinese ancestry. We discovered two new susceptibility loci for ALS at 1q32 (CAMK1G, rs6703183, Pcombined = 2.92 × 10−8, odds ratio (OR) = 1.31) and 22p11 (CABIN1 and SUSD2, rs8141797, Pcombined = 2.35 × 10−9, OR = 1.52). These two loci explain 12.48% of the overall variance in disease risk in the Han Chinese population. We found no association evidence for the previously reported loci in the Han Chinese population, suggesting genetic heterogeneity of disease susceptibility for ALS between ancestry groups. Our study identifies two new susceptibility loci and suggests new pathogenic mechanisms of ALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
09 May 2013
In the version of this article initially published online, the affiliations of authors Xiuxiu Liu, Xiaogang Li, Nan Zhang and Na Liu were incorrect. The correct affiliation of these authors is the Department of Neurology, Peking University Third Hospital, Beijing, China. The errors have been corrected in the PDF, HTML and print versions of this article.
References
Hardiman, O., van den Berg, L.H. & Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 639–649 (2011).
Cleveland, D.W. & Rothstein, J.D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819 (2001).
Bruijn, L.I., Miller, T.M. & Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (2004).
Dunckley, T. et al. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N. Engl. J. Med. 357, 775–788 (2007).
van Es, M.A. et al. ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 6, 869–877 (2007).
van Es, M.A. et al. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 40, 29–31 (2008).
van Es, M.A. et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 41, 1083–1087 (2009).
Garber, K. Genetics. The elusive ALS genes. Science 319, 20 (2008).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Takemoto-Kimura, S. et al. Molecular cloning and characterization of CLICK-III/CaMKIγ, a novel membrane-anchored neuronal Ca2+/calmodulin-dependent protein kinase (CaMK). J. Biol. Chem. 278, 18597–18605 (2003).
Nishimura, H. et al. Cloning, characterization and expression of two alternatively splicing isoforms of Ca2+/calmodulin-dependent protein kinase Iγ in the rat brain. J. Neurochem. 85, 1216–1227 (2003).
Wayman, G.A., Lee, Y.S., Tokumitsu, H., Silva, A.J. & Soderling, T.R. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron 59, 914–931 (2008).
Takemoto-Kimura, S. et al. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIγ. Neuron 54, 755–770 (2007).
Yanpallewar, S.U., Barrick, C.A., Buckley, H., Becker, J. & Tessarollo, L. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS ONE 7, e39946 (2012).
Yano, S., Tokumitsu, H. & Soderling, T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396, 584–587 (1998).
Sugahara, T. et al. Isolation of a novel mouse gene, mSVS-1/SUSD2, reversing tumorigenic phenotypes of cancer cells in vitro. Cancer Sci. 98, 900–908 (2007).
Sugahara, T. et al. von Willebrand factor type D domain mutant of SVS-1/SUSD2, vWDm, induces apoptosis in HeLa cells. Cancer Sci. 98, 909–915 (2007).
Uo, T., Veenstra, T.D. & Morrison, R.S. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J. Neurosci. 29, 2824–2832 (2009).
González de Aguilar, J.L. et al. Alteration of the Bcl-x/Bax ratio in a transgenic mouse model of amyotrophic lateral sclerosis: evidence for the implication of the p53 signaling pathway. Neurobiol. Dis. 7, 406–415 (2000).
Sathasivam, S. & Shaw, P.J. Apoptosis in amyotrophic lateral sclerosis—what is the evidence? Lancet Neurol. 4, 500–509 (2005).
Hyman, B.T. & Yuan, J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat. Rev. Neurosci. 13, 395–406 (2012).
Soo, K.Y. et al. Bim links ER stress and apoptosis in cells expressing mutant SOD1 associated with amyotrophic lateral sclerosis. PLoS ONE 7, e35413 (2012).
Guégan, C., Vila, M., Rosoklija, G., Hays, A.P. & Przedborski, S. Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J. Neurosci. 21, 6569–6576 (2001).
Forbes, R.B., Colville, S. & Swingler, R.J. Are the El Escorial and Revised El Escorial criteria for ALS reproducible? A study of inter-observer agreement. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2, 135–138 (2001).
Zhang, X.J. et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat. Genet. 41, 205–210 (2009).
Han, J.W. et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat. Genet. 41, 1234–1237 (2009).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Acknowledgements
We thank all participants in this study and all neurologists at relevant hospitals for their help in the recruitment of subjects, including Peking University Third Hospital and the First Affiliated Hospital of Anhui Medical University. This study was funded by the Key Project of the National Natural Science Foundation of China (91232717), the National Basic Research Program of China (2011CB707805), the National Natural Science Foundation of China (81171273, 31000528, 81100806, 31100812, 31000503 and 81070877) and the Special Research Fund for the Doctoral Program of Higher Education (20113420110001) and was also supported by the National Nature Science Foundation of China (31171048, 81072374, 30973043 and 30700906), the Science and Technology New Star Funds of Beijing (2007A008 and 2009A04), the Beijing Science Foundation (7112146 and 7102159), the Beijing Nova Program (2009A04 and 2007A008) and A*STAR of Singapore.
Author information
Authors and Affiliations
Contributions
K.W. and M.D. conceived this study. X. Zhang, K.W. and M.D. provided financial support. K.W., M.D., L.W., X. Zuo, Y.T. and X.J. designed the study. P.H., H.W., H.M., T.M.C.L. and Y.M. took part in the design of the study and in sample selection. M.D., Y.T., X.J., Yongzhu Han, Yongsheng Han, X. Lv, Y.F., H.Y., C.X., J.D., D.X., C.Z., F.Y., R.Y., Q.Z., C.L., B.T., X. Li, N. Zhou, Huaidong Cheng, W.D., Fangfang Zhang, J.E.L., S.Z., C.W., Y.W., J.W., X.C., Z.S., N. Zhang, X. Liu, Y.J., P.X., Z.Z., L. Shen, X.W. and N.L. conducted sample selection and data management. L.W., F.X., G.C., Y.C., Hui Cheng and X.J. collected phenotype data for the GWAS. L.W., F. Zhou, X.Y., Y.G. and X. Li collected phenotype data for the validation stage. L.H.v.d.B., J.H.V., W.R., J.R., P.M.A., A.A.-C. and C.S. collected the phenotype data for the validation stage in individuals of European ancestry. X. Zuo, B.L., X. Zheng and L.W. collected phenotype data, undertook related data handling and calculation, managed recruitment and obtained biological samples. X. Zuo, S.Y., L. Sun, Fengyu Zhang, J.L., S.X. and J.R. undertook data checking, statistical analysis and bioinformatics analyses. K.W., X. Zhang, X. Zuo, X.J. and M.D. were responsible for project management. L.S. and J.L. helped to revise the manuscript. All authors contributed to the final manuscript. K.W., M.D., L.W., X. Zuo, Y.T., X.J., L.S. and X.Z. had a key role in the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–9, Supplementary Figures 1–4 (PDF 5179 kb)
Rights and permissions
About this article
Cite this article
Deng, M., Wei, L., Zuo, X. et al. Genome-wide association analyses in Han Chinese identify two new susceptibility loci for amyotrophic lateral sclerosis. Nat Genet 45, 697–700 (2013). https://doi.org/10.1038/ng.2627
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2627
This article is cited by
-
Genetics of amyotrophic lateral sclerosis: seeking therapeutic targets in the era of gene therapy
Journal of Human Genetics (2023)
-
Transcriptomopathies of pre- and post-symptomatic frontotemporal dementia-like mice with TDP-43 depletion in forebrain neurons
Acta Neuropathologica Communications (2019)
-
Association of ATXN2 intermediate-length CAG repeats with amyotrophic lateral sclerosis correlates with the distributions of normal CAG repeat alleles among individual ethnic populations
neurogenetics (2019)
-
UNC13A variant rs12608932 is associated with increased risk of amyotrophic lateral sclerosis and reduced patient survival: a meta-analysis
Neurological Sciences (2019)
-
Meta-analysis of the association between ZNF512B polymorphism rs2275294 and risk of amyotrophic lateral sclerosis
Neurological Sciences (2018)