Abstract
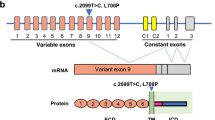
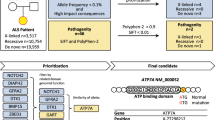
Amyotrophic lateral sclerosis 2 (ALS2) is an autosomal recessive form of juvenile ALS and has been mapped to human chromosome 2q33. Here we report the identification of two independent deletion mutations linked to ALS2 in the coding exons of the new gene ALS2. These deletion mutations result in frameshifts that generate premature stop codons. ALS2 is expressed in various tissues and cells, including neurons throughout the brain and spinal cord, and encodes a protein containing multiple domains that have homology to RanGEF as well as RhoGEF. Deletion mutations are predicted to cause a loss of protein function, providing strong evidence that ALS2 is the causative gene underlying this form of ALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Siddique, T., Nijhawan, D. & Hentati, A. Molecular genetic basis of familial ALS. Neurology 47, S27–S35 (1996).
Siddique, T. et al. Linkage of a gene causing familial amyotrophic lateral sclerosis to chromosome 21 and evidence of genetic-locus heterogeneity. N. Engl. J. Med. 324, 1381–1384 (1991).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Chance, P.F. et al. Linkage of the gene for an autosomal dominant form of juvenile amyotrophic lateral sclerosis to chromosome 9q34. Am. J. Hum. Genet. 62, 633–640 (1998).
Blair, I.P. et al. A gene for autosomal dominant juvenile amyotrophic lateral sclerosis (ALS4) localizes to a 500-kb interval on chromosome 9q34. Neurogenetics 3, 1–6 (2000).
Hentati, A. et al. Linkage of recessive familial amyotrophic lateral sclerosis to chromosome 2q33–q35. Nature Genet. 7, 425–428 (1994).
Hosler, B.A. et al. Refined mapping and characterization of the recessive familial amyotrophic lateral sclerosis locus (ALS2) on chromosome 2q33. Neurogenetics 2, 34–42 (1998).
Hentati, A. et al. Linkage of a commoner form of recessive amyotrophic lateral sclerosis to chromosome 15q15-q22 markers. Neurogenetics 2, 55–60 (1998).
Hosler, B.A. et al. Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21-q22. JAMA 284, 1664–1669 (2000).
Ben Hamida, M., Hentati, F. & Ben Hamida, C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Brain 113, 347–363 (1990).
Hadano, S. et al. A yeast artificial chromosome-based physical map of the juvenile amyotrophic lateral sclerosis (ALS2) critical region on human chromosome 2q33–q34. Genomics 55, 106–112 (1999).
Hadano, S. et al. Cloning and characterization of three novel genes, ALS2CR1, ALS2CR2, and ALS2CR3, in the juvenile amyotrophic lateral sclerosis (ALS2) critical region at chromosome 2q33–q34: candidate genes for ALS2. Genomics 71, 200–213 (2001).
Ohtsubo, M. et al. Isolation and characterization of the active cDNA of the human cell cycle gene (RCC1) involved in the regulation of onset of chromosome condensation. Genes Dev. 1, 585–593 (1987).
Luo, L. Rho GTPases in neuronal morphogenesis. Nature Rev. Neurosci. 1, 173–180 (2000).
Lerman-Sagie, T., Filiano, J., Smith, D.W. & Korson, M. Infantile onset of hereditary ascending spastic paralysis with bulbar involvement. J. Child Neurol. 11, 54–57 (1996).
Nagase, T., Kikuno, R., Nakayama, M., Hirosawa, M. & Ohara, O. Prediction of the coding sequences of unidentified human genes. XVIII. The complete sequences of 100 new cDNA clones from brain which code for large protein in vitro. DNA Res. 7, 273–281 (2000).
Meindl, A. et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nature Genet. 13, 35–42 (1996).
Renault, L. et al. The 1.7 Å crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature 392, 97–101 (1998).
Soisson, S.M., Nimnual, A.S., Uy, M., Bar-Sagi, D. & Kuriyan, J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell 95, 259–268 (1998).
Hama, H., Tall, G. G. & Horazdovsky, B.F. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J. Biol. Chem. 274, 15284–15291 (1999).
Horiuchi, H. et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 (1997).
Tall, G.G., Barbieri, M.A., Stahl, P.D. & Horazdovsky, B.F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82 (2001).
Takeshima, H., Kamazaki, S., Nishi, M., Iino, M. & Kangawa, K. Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22 (2000).
Carazo-Salas, R.E. et al. Generation of GTP-bound Ran by RCC1 is required for chromatin mitotic spindle formation. Nature 400, 178–181 (1999).
Barrett, K., Leptin, M. & Settleman, J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905–915 (1997).
Martijn, F.B.G. et al. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J. Cell Biol. 137, 1603–1613 (1997).
Roepman, R. et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum. Mol. Genet. 9, 2095–2105 (2000).
Alam, M.R. et al. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem. 272, 12667–12675 (1997).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Culbertson, M.R. RNA surveillance. Unforeseen consequences for gene expression, inherited genetic disorders and cancer. Trends Genet. 15, 74–80 (1999).
Collard, J.-F., Côté, F. & Julien, J.-P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature 375, 61–64 (1995).
Williamson, T.L. & Cleveland, D.W. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nature Neurosci. 2, 50–56 (1999).
Altschul, S.F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Matsumoto, K. et al. Neuronal apoptosis inhibitory protein (NAIP) may enhance the survival of granulosa cells thus indirectly affecting oocyte survival. Mol. Repod. Dev. 54, 103–111 (1999).
Acknowledgements
We thank the patients and other members of the ALS2 families for participating in this study; A.E. MacKenzie, R.G. Korneluk, I. Kanazawa, J.-C. Barbot and J.-C. Kaplan for providing control DNA samples; D. Rochefort for technical support; and all the members of our laboratories for helpful discussion and suggestions. This work was funded by the Japan Science and Technology Corporation, the Canadian Genetic Diseases Network, the Centre for Molecular Medicine and Therapeutics, the Nakabayashi Trust For ALS Research, the Japan Brain Foundation, the Grant-in-Aid for Scientific Research Priority Areas for the Ministry of Education, Science, Sports, and Culture (Japan), the Canadian Institute for Health Research, the Muscular Dystrophy Association (USA), the Amyotrophic Lateral Sclerosis Association, the National Institute for Neurological Disease (USA), National Institute for Aging (USA), Angel Fund for ALS Research (Boston, USA), Project ALS (New York, USA), Pierre L. DeBourgknecht ALS Research Foundation and Al-Athel ALS Foundation. M.R.H. is a holder of a Canada Research Chair. G.A.R. is supported by the Canadian Institute of Health Research. S.W.S. is a Scholar of the Medical Research Council of Canada. J.-E.I. is a scientific director of NeuroGenes/International Cooperative Research Project/Japan Science and Technology Corporation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hadano, S., Hand, C., Osuga, H. et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet 29, 166–173 (2001). https://doi.org/10.1038/ng1001-166
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1001-166
This article is cited by
-
Clinical features and molecular genetic investigation of infantile-onset ascending hereditary spastic paralysis (IAHSP) in two Chinese siblings caused by a novel splice site ALS2 variation
BMC Medical Genomics (2024)
-
Genetics of amyotrophic lateral sclerosis: seeking therapeutic targets in the era of gene therapy
Journal of Human Genetics (2023)
-
Unraveling the Potential of EphA4: A Breakthrough Target and Beacon of Hope for Neurological Diseases
Cellular and Molecular Neurobiology (2023)
-
Genomics of perivascular space burden unravels early mechanisms of cerebral small vessel disease
Nature Medicine (2023)
-
Modelling amyotrophic lateral sclerosis in rodents
Nature Reviews Neuroscience (2022)