Abstract
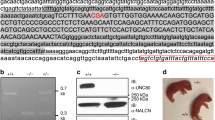
Potassium channel mutations have been described in episodic neurological diseases1. We report that K+ channel mutations cause disease phenotypes with neurodevelopmental and neurodegenerative features. In a Filipino adult-onset ataxia pedigree, the causative gene maps to 19q13, overlapping the SCA13 disease locus described in a French pedigree with childhood-onset ataxia and cognitive delay2. This region contains KCNC3 (also known as Kv3.3), encoding a voltage-gated Shaw channel with enriched cerebellar expression3. Sequencing revealed two missense mutations, both of which alter KCNC3 function in Xenopus laevis expression systems. KCNC3R420H, located in the voltage-sensing domain4, had no channel activity when expressed alone and had a dominant-negative effect when co-expressed with the wild-type channel. KCNC3F448L shifted the activation curve in the negative direction and slowed channel closing. Thus, KCNC3R420H and KCNC3F448L are expected to change the output characteristics of fast-spiking cerebellar neurons, in which KCNC channels confer capacity for high-frequency firing. Our results establish a role for KCNC3 in phenotypes ranging from developmental disorders to adult-onset neurodegeneration and suggest voltage-gated K+ channels as candidates for additional neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Graves, T.D. & Hanna, M.G. Neurological channelopathies. Postgrad. Med. J. 81, 20–32 (2005).
Herman-Bert, A. et al. Mapping of spinocerebellar ataxia 13 to chromosome 19q13.3-q13.4 in a family with autosomal dominant cerebellar ataxia and mental retardation. Am. J. Hum. Genet. 67, 229–235 (2000).
Ghanshani, S. et al. Genomic organization, nucleotide sequence, and cellular distribution of a Shaw-related potassium channel gene, Kv3.3, and mapping of Kv3.3 and Kv3.4 to human chromosomes 19 and 1. Genomics 12, 190–196 (1992).
Aggarwal, S.K. & MacKinnon, R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16, 1169–1177 (1996).
Pulst, S.M. Inherited ataxias. in Genetics of Movement Disorders (ed. Pulst, S.M.) Ch. 2 (Academic, San Diego, 2003).
Schöls, L., Bauer, P., Schmidt, T., Schulte, T. & Riess, O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 3, 291–304 (2004).
Waters, M.F. et al. An autosomal dominant ataxia maps to 19q13: allelic heterogeneity of SCA13 or novel locus? Neurology 65, 1111–1113 (2005).
Rudy, B. & McBain, C.J. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526 (2001).
Shen, N.V. & Pfaffinger, P.J. Molecular recognition and assembly sequences involved in the subfamily-specific assembly of voltage-gated K+ channel subunit proteins. Neuron 14, 625–633 (1995).
Long, S.B., Campbell, E.B. & MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Shieh, C.C., Klemic, K.G. & Kirsch, G.E. Role of transmembrane segment S5 on gating of voltage-dependent K+ channels. J. Gen. Physiol. 109, 767–778 (1997).
Smith-Maxwell, C.J., Ledwell, J.L. & Aldrich, R.W. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J. Gen. Physiol. 111, 421–439 (1998).
Seoh, S.A., Sigg, D., Papazian, D.M. & Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167 (1996).
Martina, M., Yao, G.L. & Bean, B.P. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J. Neurosci. 23, 5698–5707 (2003).
Matsukawa, H., Wolf, A., Matsushita, S., Joho, R. & Knöpfel, T. Motor dysfunction and altered synaptic transmission at the parallel fiber-Purkinje cell synapse in mice lacking potassium channels Kv3.1 and Kv3.3. J. Neurosci. 23, 7677–7684 (2003).
McMahon, A. et al. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur. J. Neurosci. 19, 3317–3327 (2004).
McKay, B.E. & Turner, R.W. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur. J. Neurosci. 20, 729–739 (2004).
Goldman-Wohl, D.S., Chan, E., Baird, D. & Heintz, N. Kv3.3b: a novel Shaw type potassium channel expressed in terminally differentiated cerebellar Purkinje cells and deep cerebellar nuclei. J. Neurosci. 14, 511–522 (1994).
Weiser, M. et al. Differential expression of Shaw-related K+ channels in the rat central nervous system. J. Neurosci. 14, 949–972 (1994).
Espinosa, F. et al. Alcohol hypersensitivity, increased locomotion, and spontaneous myoclonus in mice lacking the potassium channels Kv3.1 and Kv3.3. J. Neurosci. 21, 6657–6665 (2001).
Ruppersberg, J.P. et al. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature 352, 711–714 (1991).
Vega-Saenz de Miera, E. & Rudy, B. Modulation of K+ channels by hydrogen peroxide (1992). Biochem. Biophys. Res. Commun. 186, 1681–1687 (1992).
Duprat, F. Susceptibility of cloned K+ channels to reactive oxygen species. Proc. Natl. Acad. Sci. USA 92, 11796–11800 (1995).
McKay, B.E. & Turner, R.W. Physiological and morphological development of the rat cerebellar Purkinje cell. J. Physiol. 567, 829–850 (2005).
Ariano, M.A. et al. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J. Neurophysiol. 93, 2565–2574 (2005).
Angulo, E. et al. Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer's disease. J. Neurochem. 91, 547–557 (2004).
Baranauskas, G., Tkatch, T. & Surmeier, D.J. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K(+) channels. J. Neurosci. 15, 6394–6404 (1999).
Silverman, W.R., Tang, C.Y., Mock, A.F., Huh, K.B. & Papazian, D.M. Mg2+ modulates voltage-dependent activation in ether-a-go-go potassium channels by binding between transmembrane segments S2 and S3. J. Gen. Physiol. 116, 663–677 (2000).
Rae, J.L. & Shepard, A.R. Kv3.3 potassium channels in lens epithelium and corneal endothelium. Exp. Eye Res. 70, 339–348 (2000).
Schulteis, C.T., Nagaya, N. & Papazian, D.M. Subunit folding and assembly steps are interspersed during Shaker potassium channel biogenesis. J. Biol. Chem. 273, 26210–26217 (1998).
Acknowledgements
We thank J.L. Rae for providing the Kv3.3 cDNA clone and T. Otis, L. Timpe and F. Schweizer for manuscript critique. Technical assistance was provided by V. Garibyan, A. Camuzat and N. Benammar. This work was supported in part by US National Institutes of Health grants to S.P. (R01N533123) and D.P. (R01GM43459, R01GM66686), a National Ataxia Foundation Grant to S.P., funding from the Programme Hospitalier de Recherche Clinique (AOMO3059) to A.D., the Verum Foundation to A.B., and the EuroSCA Integrated Project (LSHM-CT-2004-503304) to A.D., A.B. and G.S. M.F.W. is supported by the American Academy of Neurology Raymond D. Adams Fellowship in Neurogenetics. N.A.M. was supported by T32GM065823.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Filipino pedigree with autosomal dominant spinocerebellar ataxia. (PDF 134 kb)
Supplementary Table 1
Primer sequences for amplification of marker MF1. (PDF 32 kb)
Rights and permissions
About this article
Cite this article
Waters, M., Minassian, N., Stevanin, G. et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 38, 447–451 (2006). https://doi.org/10.1038/ng1758
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1758
This article is cited by
-
Epilepsy as the symptom of a spinocerebellar ataxia 13 in a patient presenting with a mutation in the KCNC3 gene
BMC Neurology (2023)
-
Potential Benefit of Channel Activators in Loss-of-Function Primary Potassium Channelopathies Causing Heredoataxia
The Cerebellum (2023)
-
Timing is everything: structural insights into the disease-linked Kv3 channels controlling fast action-potential firing in the brain
Nature Communications (2022)
-
A novel giant non-cholinergic striatal interneuron restricted to the ventrolateral striatum coexpresses Kv3.3 potassium channel, parvalbumin, and the vesicular GABA transporter
Molecular Psychiatry (2022)
-
Cerebellar Kv3.3 potassium channels activate TANK-binding kinase 1 to regulate trafficking of the cell survival protein Hax-1
Nature Communications (2021)