Abstract
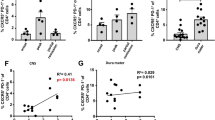
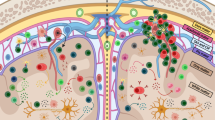
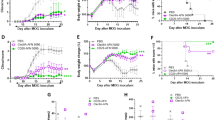
Interferon-β (IFN-β) is the major treatment for multiple sclerosis. However, this treatment is not always effective. Here we have found congruence in outcome between responses to IFN-β in experimental autoimmune encephalomyelitis (EAE) and relapsing-remitting multiple sclerosis (RRMS). IFN-β was effective in reducing EAE symptoms induced by T helper type 1 (TH1) cells but exacerbated disease induced by TH17 cells. Effective treatment in TH1-induced EAE correlated with increased interleukin-10 (IL-10) production by splenocytes. In TH17-induced disease, the amount of IL-10 was unaltered by treatment, although, unexpectedly, IFN-β treatment still reduced IL-17 production without benefit. Both inhibition of IL-17 and induction of IL-10 depended on IFN-γ. In the absence of IFN-γ signaling, IFN-β therapy was ineffective in EAE. In RRMS patients, IFN-β nonresponders had higher IL-17F concentrations in serum compared to responders. Nonresponders had worse disease with more steroid usage and more relapses than did responders. Hence, IFN-β is proinflammatory in TH17-induced EAE. Moreover, a high IL-17F concentration in the serum of people with RRMS is associated with nonresponsiveness to therapy with IFN-β.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arnason, B.G. Immunologic therapy of multiple sclerosis. Annu. Rev. Med. 50, 291–302 (1999).
Wang, A.G., Lin, Y.C., Wang, S.J., Tsai, C.P. & Yen, M.Y. Early relapse in multiple sclerosis–associated optic neuritis following the use of interferon β-1a in Chinese patients. Jpn. J. Ophthalmol. 50, 537–542 (2006).
Benveniste, E.N. & Qin, H. Type I interferons as anti-inflammatory mediators. Sci. STKE 2007, pe70 (2007).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008).
Guo, B., Chang, E.Y. & Cheng, G. The type I IFN induction pathway constrains TH17-mediated autoimmune inflammation in mice. J. Clin. Invest. 118, 1680–1690 (2008).
Nagai, T., Devergne, O., van Seventer, G.A. & van Seventer, J.M. Interferon-β mediates opposing effects on interferon-γ–dependent interleukin-12 p70 secretion by human monocyte-derived dendritic cells. Scand. J. Immunol. 65, 107–117 (2007).
McRae, B.L., Semnani, R.T., Hayes, M.P., van Seventer, G.A. & Type, I. IFNs inhibit human dendritic cell IL-12 production and TH1 cell development. J. Immunol. 160, 4298–4304 (1998).
Martín-Saavedra, F.M., Gonzalez-Garcia, C., Bravo, B. & Ballester, S. β interferon restricts the inflammatory potential of CD4+ cells through the boost of the TH2 phenotype, the inhibition of TH17 response and the prevalence of naturally occurring T regulatory cells. Mol. Immunol. 45, 4008–4019 (2008).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Fitzgerald, D.C. et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27–stimulated T cells. Nat. Immunol. 8, 1372–1379 (2007).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17–producing T cells. Immunity 24, 179–189 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
McGeachy, M.J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17–producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Platanias, L.C. Mechanisms of type-I- and type-II-interferon–mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Nguyen, K.B. et al. Interferon α/β–mediated inhibition and promotion of interferon γ: STAT1 resolves a paradox. Nat. Immunol. 1, 70–76 (2000).
Tanabe, Y. et al. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-α β responses in T lymphocytes. J. Immunol. 174, 609–613 (2005).
Wong, L.H., Hatzinisiriou, I., Devenish, R.J. & Ralph, S.J. IFN-γ priming up-regulates IFN-stimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFN-resistant melanoma cells to type I IFNs. J. Immunol. 160, 5475–5484 (1998).
McGeachy, M.J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Berenson, L.S., Gavrieli, M., Farrar, J.D., Murphy, T.L. & Murphy, K.M. Distinct characteristics of murine STAT4 activation in response to IL-12 and IFN-α. J. Immunol. 177, 5195–5203 (2006).
Parronchi, P. et al. IL-4 and IFN (α and γ) exert opposite regulatory effects on the development of cytolytic potential by TH1 or TH2 human T cell clones. J. Immunol. 149, 2977–2983 (1992).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Graber, J.J. et al. Cytokine changes during interferon-β therapy in multiple sclerosis: correlations with interferon dose and MRI response. J. Neuroimmunol. 185, 168–174 (2007).
Bartosik-Psujek, H. & Stelmasiak, Z. The interleukin-10 levels as a potential indicator of positive response to interferon β treatment of multiple sclerosis patients. Clin. Neurol. Neurosurg. 108, 644–647 (2006).
Awasthi, A. et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389 (2007).
Harrington, L.E. et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Haak, S. et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 119, 61–69 (2009).
Lucchinetti, C.F. et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125, 1450–1461 (2002).
Hengstman, G.J., Wesseling, P., Frenken, C.W. & Jongen, P.J. Neuromyelitis optica with clinical and histopathological involvement of the brain. Mult. Scler. 13, 679–682 (2007).
Zhang, Z. et al. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine 46, 79–91 (2009).
Liang, S.C. et al. An IL-17F/A heterodimer protein is produced by mouse TH17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799 (2007).
Smith, E. et al. IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J. Immunol. 179, 8274–8279 (2007).
Ishizu, T. et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain 128, 988–1002 (2005).
Warabi, Y., Matsumoto, Y. & Hayashi, H. Interferon β-1b exacerbates multiple sclerosis with severe optic nerve and spinal cord demyelination. J. Neurol. Sci. 252, 57–61 (2007).
Shimizu, Y. et al. Development of extensive brain lesions following interferon β therapy in relapsing neuromyelitis optica and longitudinally extensive myelitis. J. Neurol. 255, 305–307 (2008).
Eisen, M.B., Spellman, P.T., Brown, P.O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 (1998).
Acknowledgements
We would like to thank J. Mountz (University of Alabama at Birmingham) for the kind gift of Rebif and Y. Rosenberg-Hasson for running the multiplex cytokine assay. This study was funded by US National Institutes of Health grant R01NS 55997 to L.S., US National Multiple Sclerosis Society grant RG3891-A1, National Institutes of Health grant RO1AI1076562-01 to C.R. and National Multiple Sclerosis Society grant FG 1817-A-1 to R.C.A.
Author information
Authors and Affiliations
Contributions
R.C.A., L.S. and C.R. discussed, designed and wrote this report; L.S. and C.R. contributed equally as senior authors, and R.C.A. conducted or supervised all experiments. C.H.P., J.K. and L.F.v.d.V. characterized RRMS clinical data and collected serum. B.A.d.J., M.H. and I.K. assisted in analyzing multiplex data from RRMS. P.D., R.N., J.G.C., I.K. and R.M. assisted in EAE experiments. B.A.d.J. and L.K. performed histology on EAE spinal cords. A.C. and F.Z. performed the STAT1 activation assays. R.B., J.G.C. and A.C. assisted with mouse T cell experiments. R.d.W.M. and K.B. performed the human T cell experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Table 1 and Supplementary Methods (PDF 681 kb)
Rights and permissions
About this article
Cite this article
Axtell, R., de Jong, B., Boniface, K. et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat Med 16, 406–412 (2010). https://doi.org/10.1038/nm.2110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2110
This article is cited by
-
Transplantation of human adipose-derived stem cells overexpressing LIF/IFN-β promotes recovery in experimental autoimmune encephalomyelitis (EAE)
Scientific Reports (2022)
-
Early-life-trauma triggers interferon-β resistance and neurodegeneration in a multiple sclerosis model via downregulated β1-adrenergic signaling
Nature Communications (2021)
-
Vitamin D and lumisterol derivatives can act on liver X receptors (LXRs)
Scientific Reports (2021)
-
Neutrophil-selective deletion of Cxcr2 protects against CNS neurodegeneration in a mouse model of multiple sclerosis
Journal of Neuroinflammation (2020)
-
Sema4A is implicated in the acceleration of Th17 cell-mediated neuroinflammation in the effector phase
Journal of Neuroinflammation (2020)