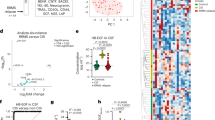
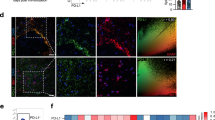
Abstract
Astrocytes have complex roles in health and disease, thus it is important to study the pathways that regulate their function. Here we report that lactosylceramide (LacCer) synthesized by β-1,4-galactosyltransferase 6 (B4GALT6) is upregulated in the central nervous system (CNS) of mice during chronic experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS). LacCer acts in an autocrine manner to control astrocyte transcriptional programs that promote neurodegeneration. In addition, LacCer in astrocytes controls the recruitment and activation of microglia and CNS-infiltrating monocytes in a non–cell autonomous manner by regulating production of the chemokine CCL2 and granulocyte-macrophage colony–stimulating factor (GM-CSF), respectively. We also detected high B4GALT6 gene expression and LacCer concentrations in CNS MS lesions. Inhibition of LacCer synthesis in mice suppressed local CNS innate immunity and neurodegeneration in EAE and interfered with the activation of human astrocytes in vitro. Thus, B4GALT6 regulates astrocyte activation and is a potential therapeutic target for MS and other neuroinflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Clarke, L.E. & Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 (2013).
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K. & Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555 (2008).
Seifert, G., Schilling, K. & Steinhauser, C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat. Rev. Neurosci. 7, 194–206 (2006).
Tsai, H.H. et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362 (2012).
Bush, T.G. et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, 297–308 (1999).
Myer, D.J., Gurkoff, G.G., Lee, S.M., Hovda, D.A. & Sofroniew, M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129, 2761–2772 (2006).
Toft-Hansen, H., Fuchtbauer, L. & Owens, T. Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia 59, 166–176 (2011).
Voskuhl, R.R. et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 29, 11511–11522 (2009).
Mayo, L., Quintana, F.J. & Weiner, H.L. The innate immune system in demyelinating disease. Immunol. Rev. 248, 170–187 (2012).
Nylander, A. & Hafler, D.A. Multiple sclerosis. J. Clin. Invest. 122, 1180–1188 (2012).
Weiner, H.L. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann. Neurol. 65, 239–248 (2009).
Joseph, J., Bittner, S., Kaiser, F.M., Wiendl, H. & Kissler, S. IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J. Immunol. 188, 216–221 (2012).
Basso, A.S. et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 118, 1532–1543 (2008).
Farez, M.F. et al. Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol. 10, 958–964 (2009).
Cao, W. et al. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity 35, 273–284 (2011).
Pluchino, S. et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422, 688–694 (2003).
Chatterjee, S. & Alsaeedi, N. Lactosylceramide synthase as a therapeutic target to mitigate multiple human diseases in animal models. Adv. Exp. Med. Biol. 749, 153–169 (2012).
Pannu, R., Won, J.S., Khan, M., Singh, A.K. & Singh, I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J. Neurosci. 24, 5942–5954 (2004).
Ajami, B., Bennett, J.L., Krieger, C., McNagny, K.M. & Rossi, F.M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Izikson, L., Klein, R.S., Charo, I.F., Weiner, H.L. & Luster, A.D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 (2000).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009).
Watkins, T.A., Emery, B., Mulinyawe, S. & Barres, B.A. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60, 555–569 (2008).
Nishie, T. β4-galactosyltransferase-5 is a lactosylceramide synthase essential for mouse extra-embryonic development. Glycobiology 20, 1311–1322 (2010).
Tokuda, N. et al. β4GalT6 is involved in the synthesis of lactosylceramide with less intensity than β4GalT5. Glycobiology 23, 1175–1183 (2013).
Yan, Y. et al. CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes. Mol. Ther. 20, 1338–1348 (2012).
Lee, J.K. et al. Lactosylceramide mediates the expression of adhesion molecules in TNF-α and IFNγ-stimulated primary cultured astrocytes. Korean J. Physiol. Pharmacol. 15, 251–258 (2011).
Huang, D.R., Wang, J., Kivisakk, P., Rollins, B.J. & Ransohoff, R.M. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193, 713–726 (2001).
Heppner, F.L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
David, S. & Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 (2011).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Murray, P.J. & Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Ponomarev, E.D. et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178, 39–48 (2007).
Gong, N., Wei, H., Chowdhury, S.H. & Chatterjee, S. Lactosylceramide recruits PKCα/ɛ and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells. Proc. Natl. Acad. Sci. USA 101, 6490–6495 (2004).
Nakamura, H. et al. Lactosylceramide interacts with and activates cytosolic phospholipase A2α. J. Biol. Chem. 288, 23264–23272 (2013).
Ho, P.P. et al. Identification of naturally occurring fatty acids of the myelin sheath that resolve neuroinflammation. Sci. Transl. Med. 4, 137ra173 (2012).
Jahng, A. et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 (2004).
Kanter, J.L. et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 12, 138–143 (2006).
Quintana, F.J. et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 105, 18889–18894 (2008).
Quintana, F.J., Yeste, A., Weiner, H.L. & Covacu, R. Lipids and lipid-reactive antibodies as biomarkers for multiple sclerosis. J. Neuroimmunol. 248, 53–57 (2012).
Schwab, J.M., Chiang, N., Arita, M. & Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 (2007).
Nolte, C. et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86 (2001).
Cardona, A.E., Huang, D., Sasse, M.E. & Ransohoff, R.M. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 1, 1947–1951 (2006).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Prinz, M., Priller, J., Sisodia, S.S. & Ransohoff, R.M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Zamanian, J.L. et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410 (2012).
Ulitsky, I. et al. Expander: from expression microarrays to networks and functions. Nat. Protoc. 5, 303–322 (2010).
Saura, J., Tusell, J.M. & Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189 (2003).
Kunis, G. et al. IFN-γ-dependent activation of the brain's choroid plexus for CNS immune surveillance and repair. Brain 136, 3427–3440 (2013).
Menheniott, T.R., Charalambous, M. & Ward, A. Derivation of primary choroid plexus epithelial cells from the mouse. Methods Mol. Biol. 633, 207–220 (2010).
Jack, C.S. et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175, 4320–4330 (2005).
Alvarez, J.I. et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011).
Acknowledgements
The authors thank G. Losyev and D. Kozoriz for cell sorting, F. Kirchhoff (University of Saarland) for providing us with GFAP transgenic mice and G.-X. Zhang (Thomas Jefferson University) for the pLenti-GFAP-EGFP-mir30-shAct1 vector. This work was supported by grants AI075285 and AI093903 from the US National Institutes of Health, RG4111A1 and JF2161-A-5 from the National Multiple Sclerosis Society and PA0069 from the International Progressive MS Alliance (F.J.Q.) and US National Institutes Transformative Grant AG-043975 (H.W.L.); L.M. is supported by a postdoctoral fellowship (FG1941A1/2) from National Multiple Sclerosis Society.
Author information
Authors and Affiliations
Contributions
L.M., M.N., P.K., A.Y., K.K. and I.D.M. performed in vitro and in vivo experiments with murine systems; S.A.T. measured lipids; J.I.A., B.E. and A.P. provided unique MS samples; J.I.A. and L.M. performed in vitro experiments with human samples; L.M., M.B. and J.P.A. performed experiments with human astrocytes in culture; L.M. and B.P. performed bioinformatics analysis; R.B. contributed to the initial experimental design; L.M., A.P., J.P.A., H.L.W. and F.J.Q. analyzed and interpreted data; L.M. and F.J.Q. wrote the manuscript; F.J.Q. conceived and supervised the study and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–3 (PDF 4932 kb)
Rights and permissions
About this article
Cite this article
Mayo, L., Trauger, S., Blain, M. et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 20, 1147–1156 (2014). https://doi.org/10.1038/nm.3681
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3681
This article is cited by
-
Understanding immune microenvironment alterations in the brain to improve the diagnosis and treatment of diverse brain diseases
Cell Communication and Signaling (2024)
-
A repair pathway lost in multiple sclerosis provides a new drug opportunity
Nature Immunology (2024)
-
Disease-associated astrocyte epigenetic memory promotes CNS pathology
Nature (2024)
-
Deletion of voltage-gated calcium channels in astrocytes decreases neuroinflammation and demyelination in a murine model of multiple sclerosis
Journal of Neuroinflammation (2023)
-
Duhuo Jisheng decoction alleviates neuroinflammation and neuropathic pain by suppressing microglial M1 polarization: a network pharmacology research
Journal of Orthopaedic Surgery and Research (2023)