Key Points
-
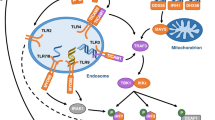
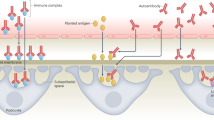
Viruses can serve as triggers of autoimmune disease by acting as adjuvants, leading to the priming of autoantigen-specific lymphocytes. Autoantigen-reactive T and B cells can also be activated following recognition of viral antigens with similarity to self antigens (molecular mimicry) or following recognition of new self epitopes released from damaged tissues (epitope spreading).
-
Virus infection and immune control can also be altered, in terms of the type of immune response, the viral set point during chronic infections and the anatomical distribution of virus-specific lymphocytes, in patients with autoimmune diseases. Antiviral responses may be modified in these patients as a consequence of the same genetic variants that predispose them to autoimmune diseases.
-
Dysregulated antiviral immune responses might reflect the contribution of these responses to autoimmune disease or might result from altered immune homeostasis in patients.
-
Possible underlying mechanisms are discussed in the setting of autoimmune inflammation of the central nervous system. We focus on recent studies on the induction of immune-mediated demyelination by Theiler's murine encephalomyelitis virus infection and the association of multiple sclerosis with increased immune control of Epstein–Barr virus.
-
An understanding of the underlying mechanisms of dysregulated virus-specific immune responses in autoimmune diseases could reveal new approaches for treating or diagnosing these diseases.
Abstract
The predisposition of individuals to several common autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis, is genetically linked to certain human MHC class II molecules and other immune modulators. However, genetic predisposition is only one risk factor for the development of these diseases, and low concordance rates in monozygotic twins, as well as the geographical distribution of disease risk, suggest the involvement of environmental factors in the development of these diseases. Among these environmental factors, infections have been implicated in the onset and/or promotion of autoimmunity. In this Review, we outline the mechanisms by which viral infection can trigger autoimmune disease and describe the pathways by which infection and immune control of infectious disease might be dysregulated during autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ishii, K. J., Koyama, S., Nakagawa, A., Coban, C. & Akira, S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3, 352–363 (2008).
Marrack, P., Scott-Browne, J. P., Dai, S., Gapin, L. & Kappler, J. W. Evolutionarily conserved amino acids that control TCR–MHC interaction. Annu. Rev. Immunol. 26, 171–203 (2008).
Fujinami, R. S., Oldstone, M. B., Wroblewska, Z., Frankel, M. E. & Koprowski, H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc. Natl Acad. Sci. USA 80, 2346–2350 (1983).
Oldstone, M. B. Molecular mimicry and immune-mediated diseases. FASEB J. 12, 1255–1265 (1998).
Fujinami, R. S. & Oldstone, M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 230, 1043–1045 (1985). This publication introduced the concept of molecular mimicry.
Wucherpfennig, K. W. & Strominger, J. L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705 (1995).
Lang, H. L. et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature Immunol. 3, 940–943 (2002).
Gregersen, J. W. et al. Functional epistasis on a common MHC haplotype associated with multiple sclerosis. Nature 443, 574–577 (2006).
Miller, S. D. et al. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nature Med. 3, 1133–1136 (1997). The first description of a persistent virus infection that can lead to autoimmunity through epitope spreading.
Zhao, Z. S., Granucci, F., Yeh, L., Schaffer, P. A. & Cantor, H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 279, 1344–1347 (1998).
Deshpande, S. P. et al. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J. Virol. 75, 3077–3088 (2001).
Benoist, C. & Mathis, D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nature Immunol. 2, 797–801 (2001).
Christen, U. et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Invest. 114, 1290–1298 (2004).
Mokhtarian, F., Zhang, Z., Shi, Y., Gonzales, E. & Sobel, R. A. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J. Neuroimmunol. 95, 43–54 (1999).
Gauntt, C. J. et al. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J. Immunol. 154, 2983–2995 (1995).
Lawson, C. M. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell. Mol. Life Sci. 57, 552–560 (2000).
Cunningham, M. W. et al. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc. Natl Acad. Sci. USA 89, 1320–1324 (1992).
Oldstone, M. B., Nerenberg, M., Southern, P., Price, J. & Lewicki, H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65, 319–331 (1991).
Ohashi, P. S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991). References 18 and 19 provide the first evidence for the initiation of autoimmune disease following viral infection of a transgenic mouse expressing a viral protein.
von Herrath, M. G., Dockter, J. & Oldstone, M. B. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1, 231–242 (1994).
Evans, C. F., Horwitz, M. S., Hobbs, M. V. & Oldstone, M. B. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J. Exp. Med. 184, 2371–2384 (1996).
Olson, J. K., Croxford, J. L., Calenoff, M. A., Dal Canto, M. C. & Miller, S. D. A virus-induced molecular mimicry model of multiple sclerosis. J. Clin. Invest. 108, 311–318 (2001). This study provided the first description of initiation of autoimmune disease following infection with a non-pathological virus variant that was engineered to express a peptide mimic of a myelin self antigen.
Carrizosa, A. M. et al. Expansion by self antigen is necessary for the induction of experimental autoimmune encephalomyelitis by T cells primed with a cross-reactive environmental antigen. J. Immunol. 161, 3307–3314 (1998).
Croxford, J. L., Ercolini, A. M., Degutes, M. & Miller, S. D. Structural requirements for initiation of cross-reactivity and CNS autoimmunity with a PLP139–151 mimic peptide derived from murine hepatitis virus. Eur. J. Immunol. 36, 2671–2680 (2006).
Croxford, J. L., Olson, J. K., Anger, H. A. & Miller, S. D. Initiation and exacerbation of autoimmune demyelination of the central nervous system via virus-induced molecular mimicry: implications for the pathogenesis of multiple sclerosis. J. Virol. 79, 8581–8590 (2005).
Greene, M. T., Ercolini, A. M., Degutes, M. & Miller, S. D. Differential induction of experimental autoimmune encephalomyelitis by myelin basic protein molecular mimics in mice humanized for HLA-DR2 and an MBP(85–99)-specific T cell receptor. J. Autoimmun. 31, 399–407 (2008).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Samuels, J., Ng, Y. S., Coupillaud, C., Paget, D. & Meffre, E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201, 1659–1667 (2005).
Lovett-Racke, A. E. et al. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J. Clin. Invest. 101, 725–730 (1998).
Markovic-Plese, S., Cortese, I., Wandinger, K. P., McFarland, H. F. & Martin, R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J. Clin. Invest. 108, 1185–1194 (2001).
Baranzini, S. E. et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol. 163, 5133–5144 (1999).
Babbe, H. et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404 (2000).
Skulina, C. et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl Acad. Sci. USA 101, 2428–2433 (2004).
Meinl, E. et al. Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis. Complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J. Clin. Invest. 92, 2633–2643 (1993).
Goebels, N. et al. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects: epitope spreading versus clonal persistence. Brain 123, 508–518 (2000).
Muraro, P. A. et al. Molecular tracking of antigen-specific T cell clones in neurological immune-mediated disorders. Brain 126, 20–31 (2003).
Thacker, E. L., Mirzaei, F. & Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59, 499–503 (2006). A meta-analysis of studies investigating the risk for the development of multiple sclerosis after symptomatic primary infection with EBV.
Nielsen, T. R. et al. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 64, 72–75 (2007).
Nielsen, T. et al. Effects of infectious mononucleosis and HLA-DRB1*15 in multiple sclerosis. Mult. Scler. 19 Jan 2009 (doi:10.1177/1352458508100037).
Lünemann, J. D. et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-γ and IL-2. J. Exp. Med. 205, 1763–1773 (2008). A description of the selective expansion of EBNA1-specific CD4+ T cells in patients with multiple sclerosis.
Harari, A. et al. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl Acad. Sci. USA 104, 16233–16238 (2007).
Lünemann, J. D. et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain 129, 1493–1506 (2006).
Zipris, D. et al. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J. Immunol. 174, 131–142 (2005).
Walker, L. S. & Abbas, A. K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nature Rev. Immunol. 2, 11–19 (2002).
Lehmann, P. V., Forsthuber, T., Miller, A. & Sercarz, E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358, 155–157 (1992). The initial description of epitope spreading.
McRae, B. L., Vanderlugt, C. L., Dal Canto, M. C. & Miller, S. D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 182, 75–85 (1995). The first study to show the functional and pathological importance of epitope spreading to disease progression in relapsing EAE.
Yu, M., Johnson, J. M. & Tuohy, V. K. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis for peptide-specific therapy after onset of clinical disease. J. Exp. Med. 183, 1777–1788 (1996).
Katz-Levy, Y. et al. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J. Clin. Invest. 104, 599–610 (1999).
Katz-Levy, Y. et al. Temporal development of autoreactive Th1 responses and endogenous presentation of self myelin epitopes by central nervous system-resident APCs in Theiler's virus-infected mice. J. Immunol. 165, 5304–5314 (2000).
Borrow, P. et al. Investigation of the role of delayed-type-hypersensitivity responses to myelin in the pathogenesis of Theiler's virus-induced demyelinating disease. Immunology 93, 478–484 (1998).
Kaufman, D. L. et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366, 69–72 (1993).
Wucherpfennig, K. W. Mechanisms for the induction of autoimmunity by infectious agents. J. Clin. Invest. 108, 1097–1104 (2001).
Brocke, S. et al. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature 365, 642–644 (1993).
Cole, B. C. & Griffiths, M. M. Triggering and exacerbation of autoimmune arthritis by the Mycoplasma arthritidis superantigen MAM. Arthritis Rheum. 36, 994–1002 (1993).
Dalwadi, H., Wei, B., Kronenberg, M., Sutton, C. L. & Braun, J. The Crohn's disease-associated bacterial protein I2 is a novel enteric T cell superantigen. Immunity 15, 149–158 (2001).
Sutkowski, N., Conrad, B., Thorley-Lawson, D. A. & Huber, B. T. Epstein–Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity 15, 579–589 (2001).
Tai, A. et al. Human endogenous retrovirus-K18 Env as a risk factor in multiple sclerosis. Mult. Scler. 14, 1175–1180 (2008).
Deng, G. M. & Tsokos, G. C. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. J. Immunol. 181, 4019–4026 (2008).
Pender, M. P. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 24, 584–588 (2003).
Olson, J. K., Ludovic Croxford, J. & Miller, S. D. Innate and adaptive immune requirements for induction of autoimmune demyelinating disease by molecular mimicry. Mol. Immunol. 40, 1103–1108 (2004).
Thorley-Lawson, D. A. & Gross, A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350, 1328–1337 (2004).
Nanbo, A., Inoue, K., Adachi-Takasawa, K. & Takada, K. Epstein–Barr virus RNA confers resistance to interferon-α-induced apoptosis in Burkitt's lymphoma. EMBO J. 21, 954–965 (2002).
Serafini, B. et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007). This study characterized dysregulation of EBV infection in the CNS of patients with multiple sclerosis.
Fujinami, R. S., von Herrath, M. G., Christen, U. & Whitton, J. L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 19, 80–94 (2006).
Gutcher, I. & Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Invest. 117, 1119–1127 (2007).
Hamilton-Williams, E. E. et al. Cutting edge: TLR ligands are not sufficient to break cross-tolerance to self-antigens. J. Immunol. 174, 1159–1163 (2005).
Gronski, M. A. et al. TCR affinity and negative regulation limit autoimmunity. Nature Med. 10, 1234–1239 (2004).
Lang, K. S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nature Med. 11, 138–145 (2005).
Medzhitov, R. & Janeway, C. A. Jr. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002).
Matzinger, P. An innate sense of danger. Ann. NY Acad. Sci. 961, 341–342 (2002).
Qureshi, S. T. & Medzhitov, R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 4, 87–94 (2003).
Quintana-Murci, L., Alcais, A., Abel, L. & Casanova, J. L. Immunology in natura: clinical, epidemiological and evolutionary genetics of infectious diseases. Nature Immunol. 8, 1165–1171 (2007).
Leadbetter, E. A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Leslie, D., Lipsky, P. & Notkins, A. L. Autoantibodies as predictors of disease. J. Clin. Invest. 108, 1417–1422 (2001).
Lau, C. M. et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 (2005).
Vollmer, J. et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 (2005).
Bratton, D. L. & Henson, P. M. Autoimmunity and apoptosis: refusing to go quietly. Nature Med. 11, 26–27 (2005).
Bettelli, E., Korn, T., Oukka, M. & Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
McFarland, H. F. & Martin, R. Multiple sclerosis: a complicated picture of autoimmunity. Nature Immunol. 8, 913–919 (2007).
Carlson, T., Kroenke, M., Rao, P., Lane, T. E. & Segal, B. The Th17–ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 205, 811–823 (2008).
Maloy, K. J. et al. CD4+ T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 191, 2159–2170 (2000).
Rentenaar, R. J. et al. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J. Clin. Invest. 105, 541–548 (2000).
Matsuoka, M. & Jeang, K. T. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nature Rev. Cancer 7, 270–280 (2007).
Babcock, G. J., Decker, L. L., Volk, M. & Thorley-Lawson, D. A. EBV persistence in memory B cells in vivo. Immunity 9, 395–404 (1998).
Dumais, N. et al. T-cell receptor/CD28 engagement when combined with prostaglandin E2 treatment leads to potent activation of human T-cell leukemia virus type 1. J. Virol. 77, 11170–11179 (2003).
Laichalk, L. L. & Thorley-Lawson, D. A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein–Barr virus in vivo. J. Virol. 79, 1296–1307 (2005).
Daibata, M., Speck, S. H., Mulder, C. & Sairenji, T. Regulation of the BZLF1 promoter of Epstein–Barr virus by second messengers in anti-immunoglobulin-treated B cells. Virology 198, 446–454 (1994).
Chene, A. et al. A molecular link between malaria and Epstein–Barr virus reactivation. PLoS Pathog. 3, e80 (2007).
Gross, A. J., Hochberg, D., Rand, W. M. & Thorley-Lawson, D. A. EBV and systemic lupus erythematosus: a new perspective. J. Immunol. 174, 6599–6607 (2005).
Kang, I. et al. Defective control of latent Epstein–Barr virus infection in systemic lupus erythematosus. J. Immunol. 172, 1287–1294 (2004).
Berner, B. R. et al. Phenotypic and functional analysis of EBV-specific memory CD8 cells in SLE. Cell. Immunol. 235, 29–38 (2005).
Balandraud, N. et al. Epstein–Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum. 48, 1223–1228 (2003).
Lünemann, J. D. et al. Increased frequency of EBV specific effector memory CD8+ T cells is associated with higher viral load in rheumatoid arthritis. J. Immunol. 181, 991–1000 (2008).
Appay, V. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8, 379–385 (2002).
Smedby, K. E., Baecklund, E. & Askling, J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol. Biomarkers Prev. 15, 2069–2077 (2006).
Baranzini, S. E. et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum. Mol. Genet. 18, 767–778 (2009).
Harley, J. B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature Genet. 40, 204–210 (2008).
Tamiya, G. et al. Whole genome association study of rheumatoid arthritis using 27 039 microsatellites. Hum. Mol. Genet. 14, 2305–2321 (2005).
Concannon, P. et al. A second-generation screen of the human genome for susceptibility to insulin-dependent diabetes mellitus. Nature Genet. 19, 292–296 (1998).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13–BLK and ITGAM–ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
Nath, S. K. et al. A nonsynonymous functional variant in integrin-α M (encoded by ITGAM) is associated with systemic lupus erythematosus. Nature Genet. 40, 152–154 (2008).
Hafler, D. A. et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357, 851–862 (2007).
Lundmark, F. et al. Variation in interleukin 7 receptor α chain (IL7R) influences risk of multiple sclerosis. Nature Genet. 39, 1108–1113 (2007).
Gregory, S. G. et al. Interleukin 7 receptor α chain (IL7R) shows allelic and functional association with multiple sclerosis. Nature Genet. 39, 1083–1091 (2007).
Burrows, S. R., Khanna, R., Burrows, J. M. & Moss, D. J. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein–Barr virus CTL epitope: implications for graft-versus-host disease. J. Exp. Med. 179, 1155–1161 (1994).
Apolloni, A. et al. Sequence variation of cytotoxic T cell epitopes in different isolates of Epstein–Barr virus. Eur. J. Immunol. 22, 183–189 (1992).
Bell, M. J. et al. Widespread sequence variation in Epstein–Barr virus nuclear antigen 1 influences the antiviral T cell response. J. Infect. Dis. 197, 1594–1597 (2008).
Redondo, M. J., Jeffrey, J., Fain, P. R., Eisenbarth, G. S. & Orban, T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 359, 2849–2850 (2008).
Scotet, E. et al. T cell response to Epstein–Barr virus transactivators in chronic rheumatoid arthritis. J. Exp. Med. 184, 1791–1800 (1996).
Hislop, A. D., Taylor, G. S., Sauce, D. & Rickinson, A. B. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu. Rev. Immunol. 25, 587–617 (2007).
Landais, E. et al. Direct killing of Epstein–Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 103, 1408–1416 (2004).
Scotet, E. et al. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 29, 973–985 (1999).
Krumbholz, M. et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129, 200–211 (2006).
Meyer-Bahlburg, A. & Rawlings, D. J. B cell autonomous TLR signaling and autoimmunity. Autoimmun. Rev. 7, 313–316 (2008).
Horwitz, M. S. et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nature Med. 4, 781–785 (1998). This study provides an example of virus-induced autoimmune disease involving bystander tissue damage.
Lawson, C. M., O'Donoghue, H. L. & Reed, W. D. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology 75, 513–519 (1992).
Fairweather, D., Kaya, Z., Shellam, G. R., Lawson, C. M. & Rose, N. R. From infection to autoimmunity. J. Autoimmun. 16, 175–186 (2001).
Horwitz, M. S., Ilic, A., Fine, C., Rodriguez, E. & Sarvetnick, N. Presented antigen from damaged pancreatic β cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J. Clin. Invest. 109, 79–87 (2002).
Jones, D. B. & Crosby, I. Proliferative lymphocyte responses to virus antigens homologous to GAD65 in IDDM. Diabetologia 39, 1318–1324 (1996).
Ylipaasto, P. et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet β cells. Diabetologia 47, 225–239 (2004).
Menser, M. A., Forrest, J. M. & Bransby, R. D. Rubella infection and diabetes mellitus. Lancet 1, 57–60 (1978).
Ou, D., Mitchell, L. A., Metzger, D. L., Gillam, S. & Tingle, A. J. Cross-reactive rubella virus and glutamic acid decarboxylase (65 and 67) protein determinants recognised by T cells of patients with type I diabetes mellitus. Diabetologia 43, 750–762 (2000).
Levin, M. C. et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nature Med. 8, 509–513 (2002).
Johnson, R. T. et al. Measles encephalomyelitis — clinical and immunologic studies. N. Engl. J. Med. 310, 137–141 (1984).
Jarius, S. et al. The intrathecal, polyspecific antiviral immune response: specific for MS or a general marker of CNS autoimmunity? J. Neurol. Sci. 28 Nov 2008 (doi:10.1016/j.jns.2008.08.002).
Link, H. et al. Virus-reactive and autoreactive T cells are accumulated in cerebrospinal fluid in multiple sclerosis. J. Neuroimmunol. 38, 63–73 (1992).
Ascherio, A. & Munch, M. Epstein–Barr virus and multiple sclerosis. Epidemiology 11, 220–224 (2000).
Sundstrom, P. et al. An altered immune response to Epstein–Barr virus in multiple sclerosis: a prospective study. Neurology 62, 2277–2282 (2004).
Levin, L. I. et al. Temporal relationship between elevation of Epstein–Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 293, 2493–2500 (2005).
Lünemann, J. D. et al. Broadened and elevated humoral immune responses to EBNA1 in pediatric multiple sclerosis. Neurology 71, 1033–1035 (2008).
Tosato, G., Steinberg, A. D. & Blaese, R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N. Engl. J. Med. 305, 1238–1243 (1981).
Tosato, G. et al. Abnormally elevated frequency of Epstein–Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J. Clin. Invest. 73, 1789–1795 (1984).
Alspaugh, M. A., Henle, G., Lennette, E. T. & Henle, W. Elevated levels of antibodies to Epstein–Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J. Clin. Invest. 67, 1134–1140 (1981).
McClain, M. T. et al. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature Med. 11, 85–89 (2005).
Challoner, P. B. et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl Acad. Sci. USA 92, 7440–7444 (1995).
Soldan, S. S. et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nature Med. 3, 1394–1397 (1997).
Sospedra, M. et al. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog. 1, e41 (2005).
Kozireva, S. V. et al. Incidence and clinical significance of parvovirus B19 infection in patients with rheumatoid arthritis. J. Rheumatol. 35, 1265–1270 (2008).
Saal, J. G. et al. Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol. Int. 12, 147–151 (1992).
Seve, P. et al. Lupus-like presentation of parvovirus B19 infection. Semin. Arthritis Rheum. 34, 642–648 (2005).
Acknowledgements
The laboratory of C.M. is supported by the Dana Foundation's Neuroimmunology programme, the Arnold and Mabel Beckman Foundation, the Alexandrine and Alexander Sinsheimer Foundation, the Burroughs Wellcome Fund, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health) and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital). J.D.L. is supported by a Dana Foundation and Irvington Institute's Human Immunology Fellowship, a Pilot Grant from the National Multiple Sclerosis Society (PP1145) and an Institutional Clinical and Translational Science Pilot and Collaborative Project Grant (to the Rockefeller University Hospital). The laboratory of S.D.M. and M.T.G. is supported by the National Institute for Neurological Diseases and Stroke (R01 NS-023349, R01 NS-040460 and R01 NS-030871), the National Multiple Sclerosis Society (RG 3793-A-7) and the Myelin Repair Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Pattern-recognition receptor
-
A host receptor (such as a Toll-like receptor) that can sense pathogen-associated molecular patterns and initiate signalling cascades (which involve activation of nuclear factor-κB) that lead to an innate immune response.
- Adjuvant
-
A non-infectious form of immune activation used to increase immune responses to antigen.
- Molecular mimicry
-
A term used to describe what happens when a T- or B-cell receptor recognizes a microbial peptide that is structurally similar to a self peptide. The immune response, which is initially directed at the microbial peptide, spreads to tissues that present the crossreactive self peptide, resulting in autoimmunity.
- Negative selection
-
The intrathymic elimination of double-positive or single-positive thymocytes that express T-cell receptors with high affinity for self antigens.
- Polyfunctional T cell
-
A T cell that has two or more functions, including, but not limited to, cytotoxicity and production of cytokines or chemokines. The development of multiparameter flow cytometry has facilitated the extensive analysis of T-cell effector functions at the single-cell level.
- Clonal exhaustion
-
A state of non-reactivity in which all precursor lymphocytes are induced by a persistent antigen (or antigens) to become effector cells, purging the immune-response repertoire of this specificity (or specificities).
- Activation-induced cell death
-
A process by which fully activated T cells undergo programmed cell death through engagement of cell-surface-expressed death receptors, such as CD95 (also known as FAS) or the tumour-necrosis-factor receptor.
- Bystander activation
-
Activation and/or expansion of an immune response at a site of direct inflammation-induced tissue damage.
- Epitope spreading
-
A process by which autoreactive T-cell or B-cell responses induced by a single peptide (or epitope) can spread to include other peptides (or epitopes) in the same autoantigen (intramolecular spreading) or in other self antigens (intermolecular spreading) that are released after T- or B-cell-mediated bystander tissue damage.
- Immunodominant epitope
-
A portion of an antigen that is targeted preferentially or to a greater level during an immune response.
- Superantigen
-
A microbial protein that activates all T cells which express a particular set of T-cell receptor (TCR) Vβ chains by cross-linking the TCR to a particular MHC molecule regardless of the peptide presented.
- Altered peptide ligand
-
(APL). A peptide analogue of the original antigenic peptide. APLs commonly have amino acid substitutions at T-cell receptor (TCR) contact residues. TCR engagement by these APLs usually leads to partial or incomplete T-cell activation. Antagonistic APLs can specifically antagonize and inhibit T-cell activation induced by the wild-type antigenic peptide.
- Rheumatoid factor
-
An antibody (usually IgM) that binds to the Fc region of IgG, thereby forming immune complexes. Rheumatoid factors are sometimes found in patients with rheumatoid arthritis or other autoimmune diseases, such as systemic lupus erythematosus.
Rights and permissions
About this article
Cite this article
Münz, C., Lünemann, J., Getts, M. et al. Antiviral immune responses: triggers of or triggered by autoimmunity?. Nat Rev Immunol 9, 246–258 (2009). https://doi.org/10.1038/nri2527
Issue Date:
DOI: https://doi.org/10.1038/nri2527
This article is cited by
-
Achalasia alters physiological networks depending on sex
Scientific Reports (2024)
-
Secrets and lies of host–microbial interactions: MHC restriction and trans-regulation of T cell trafficking conceal the role of microbial agents on the edge between health and multifactorial/complex diseases
Cellular and Molecular Life Sciences (2024)
-
A Pilot Study to Develop Paraneoplastic Cerebellar Degeneration Mouse Model
The Cerebellum (2023)
-
Potential role of EBV and Toll-like receptor 9 ligand in patients with systemic lupus erythematosus
Immunologic Research (2023)
-
Liposomal form of Dexamethasone in Experimental Autoimmune Uveitis: Clinical and Immunological Effects
Pharmaceutical Chemistry Journal (2023)