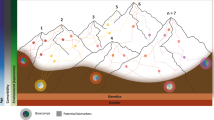
Abstract
The development of interventions to slow or prevent progression represents an important aim for current research into Parkinson disease (PD). General agreement prevails that success in this endeavor will depend on a clearer understanding of etiology and pathogenesis, and several important advances have recently been made, particularly in defining the genetic causes of PD. Studies of the biochemical consequences of the mutations that cause familial PD, and postmortem brain studies of idiopathic, sporadic PD, have highlighted mitochondrial dysfunction, oxidative stress, and protein metabolism by the ubiquitin–proteasomal and autophagy systems as being central to pathogenesis. In parallel with advances in etiopathogenesis, a clearer perception has developed of the clinical prodrome of PD, offering an opportunity to identify individuals who are at risk of PD, as well as those in the earliest clinical phase of the disease that might even precede the onset of motor symptoms. These populations are potentially the most suitable in which to test new protective therapies, and to study potential peripheral markers of disease progression. The awareness of the early symptomatic period of PD also raises the possibility of providing treatments that not only improve motor function but might also favorably modify outcome.
Key Points
-
The biochemical abnormalities that constitute the molecular prodrome of Parkinson disease (PD) can be initiated by genetic or environmental factors and seem to be common to familial and sporadic disease
-
The molecular prodrome will determine the pathology that evolves in PD and the associated cell dysfunction and death
-
The clinical prodrome will, in turn, reflect the evolving molecular and pathological prodromes and offers an important opportunity for early detection and therapeutic intervention
-
Future symptomatic and disease-modifying or neuroprotective drugs are likely to be based on interventions designed to interrupt the evolution of the molecular and pathological prodromes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schapira, A. H. Etiology of Parkinson's disease. Neurology 66 (10 Suppl. 4), S10–S23 (2006).
Gasser, T. Genomic and proteomic biomarkers for Parkinson disease. Neurology 72 (7 Suppl.), S27–S31 (2009).
Schapira, A. H. et al. Perspectives on recent advances in the understanding and treatment of Parkinson's disease. Eur. J. Neurol. 16, 1090–1099 (2009).
Tolosa, E., Gaig, C., Santamaria, J. & Compta, Y. Diagnosis and the premotor phase of Parkinson disease. Neurology 72 (7 Suppl.), S12–S20 (2009).
Chaudhuri, K. R., Healy, D. G. & Schapira, A. H. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 5, 235–245 (2006).
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M. & Morris, J. G. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844 (2008).
Chaudhuri, K. R. & Schapira, A. H. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474 (2009).
Lang, A. E. & Obeso, J. A. Challenges in Parkinson's disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 3, 309–316 (2004).
Bras, J., Singleton, A., Cookson, M. R. & Hardy, J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 275, 5767–5773 (2008).
Gupta, A., Dawson, V. L. & Dawson, T. M. What causes cell death in Parkinson's disease? Ann. Neurol. 64 (Suppl. 2), S3–S15 (2008).
Schapira, A. H. Neurobiology and treatment of Parkinson's disease. Trends Pharmacol. Sci. 30, 41–47 (2009).
Hirsch, E. C. & Hunot, S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 8, 382–397 (2009).
Martinez-Vicente, M. & Cuervo, A. M. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 6, 352–361 (2007).
Pan, T., Kondo, S., Le, W. & Jankovic, J. The role of autophagy–lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain 131, 1969–1978 (2008).
Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006).
Majeski, A. E. & Dice, J. F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36, 2435–2444 (2004).
Dice, J. F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 15, 305–309 (1990).
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004).
Singleton, A. B. et al. α-Synuclein locus triplication causes Parkinson's disease. Science 302, 841 (2003).
Fuchs, J. et al. Genetic variability in the SNCA gene influences α-synuclein levels in the blood and brain. FASEB J. 22, 1327–1334 (2008).
Chau, K. Y., Ching H. L., Schapira, A. H. & Cooper, J. M. Relationship between alpha synuclein phosphorylation, proteasomal inhibition and cell death: relevance to Parkinson's disease pathogenesis. J. Neurochem. 110, 1005–1013 (2009).
Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506 (2008).
Olanow, C. W. & Prusiner, S. B. Is Parkinson's disease a prion disorder? Proc. Natl Acad. Sci. USA 106, 12571–12572 (2009).
Schapira, A. H. et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1, 1269 (1989).
Schapira, A. H. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 7, 97–109 (2008).
Kuroda, Y. et al. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum. Mol. Genet. 15, 883–895 (2006).
Poole, A. C. et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl Acad. Sci. USA 105, 1638–1643 (2008).
Deng, H., Dodson, M. W., Huang, H. & Guo, M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl Acad. Sci. USA 105, 14503–14508 (2008).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010).
Flinn, L. et al. Complex I deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio). Brain 132, 1613–1623 (2009).
Gegg, M. E., Cooper, J. M., Schapira, A. H. & Taanman, J. W. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE 4, e4756 (2009).
Grünewald, A. et al. Differential effects of PINK1 nonsense and missense mutations on mitochondrial function and morphology. Exp. Neurol. 219, 266–273 (2009).
Gandhi, S. et al. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 33, 627–638 (2009).
Morais V. A. et al. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 1, 99–111 (2009).
Park, J. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 (2006).
Plun-Favreau, H. et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat. Cell Biol. 9, 1243–1252 (2007).
Taanman, J. W. et al. Analysis of mutant DNA polymerase γ in patients with mitochondrial DNA depletion. Hum. Mutat. 30, 248–254 (2009).
Hudson, G. et al. Mutation of the linker region of the polymerase γ-1 (POLG1) gene associated with progressive external ophthalmoplegia and parkinsonism. Arch. Neurol. 64, 553–557 (2007).
Taanman, J. W. & Schapira, A. H. Analysis of the trinucleotide CAG repeat from the DNA polymerase γ gene (POLG) in patients with Parkinson's disease. Neurosci. Lett. 376, 56–59 (2005).
Luoma, P. T. et al. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology 69, 1152–1159 (2007).
Healy, D. G. et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case–control study. Lancet Neurol. 7, 583–590 (2008).
Kim, Y. et al. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 377, 975–980 (2008).
Imai, Y. et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 27, 2432–2443 (2008).
Tain, L. S. et al. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat. Neurosci. 12, 1129–1135 (2009).
Greggio, E. et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J. Biol. Chem. 283, 16906–16914 (2008).
Plowey, E. D., Cherra, S. J., Liu, Y.-J. & Chu, C. T. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J. Neurochem. 105, 1048–1056 (2008).
Li, Y. et al. Mutant LRRK2R1441G BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat. Neurosci. 12, 826–828 (2009).
Satake, W., Nakabayashi, Y., Mizuta, I. & Hirota, Y. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 41, 1303–1307 (2009).
Simón-Sánchez, J., Schulte, C., Bras, J. M. & Sharma, M. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308–1312 (2009).
Sidransk, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Haron-Peretz, J., Rosenbaum, H. & Gershoni-Baruch, R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 351, 1972–1977 (2004).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132, 1783–1794 (2009).
Futerman, A. H. & van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5, 554–565 (2004).
Deganuto, M. et al. Altered intracellular redox status in Gaucher disease fibroblasts and impairment of adaptive response against oxidative stress. J. Cell. Physiol. 212, 223–235 (2007).
Gibb, W. R. & Lees, A. J. The progression of idiopathic Parkinson's disease is not explained by age-related changes. Clinical and pathological comparisons with post-encephalitic parkinsonian syndrome. Acta Neuropathol. 73, 195–201 (1987).
Morrish, P. K., Rakshi, J. S., Bailey, D. L., Sawle, G. V. & Brooks, D. J. Measuring the rate of progression and estimating the preclinical period of Parkinson's disease with [18F]dopa PET. J. Neurol. Neurosurg. Psychiatry 64, 314–319 (1998).
Marek, K. et al. [123I]β-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology 57, 2089–2094 (2001).
Hawkes, C. H. The prodromal phase of sporadic Parkinson's disease: does it exist and if so how long is it? Mov. Disord. 23, 1799–1807 (2008).
Katzenschlager, R. & Lees, A. J. Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr. Opin. Neurol. 17, 417–423 (2004).
Ross, G. W. et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann. Neurol. 63, 167–173 (2008).
Sommer, U. et al. Detection of presymptomatic Parkinson's disease: combining smell tests, transcranial sonography, and SPECT. Mov. Disord. 19, 1196–1202 (2004).
Ponsen, M. M. et al. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann. Neurol. 56, 173–181 (2004).
Bohnen, N. I., Studenski, S. A., Constantine, G. M. & Moore, R. Y. Diagnostic performance of clinical motor and non-motor tests of Parkinson disease: a matched case–control study. Eur. J. Neurol. 15, 685–691 (2008).
Schapira, A. H. Excessive daytime sleepiness in Parkinson's disease. Neurology 63 (8 Suppl. 3), S24–S27 (2004).
Schenck, C. H., Bundlie, S. R. & Mahowald, M. W. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 46, 388–393 (1996).
Iranzo, A. et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577 (2006).
Postuma, R. B., Lang, A. E., Massicotte-Marquez, J. & Montplaisir, J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 66, 845–851 (2006).
Stiasny-Kolster, K. et al. Combination of 'idiopathic' REM sleep behaviour disorder and olfactory dysfunction as possible indicator for α-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 128, 126–137 (2005).
Bloch, A., Probst, A., Bissig, H., Adams, H. & Tolnay, M. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol. Appl. Neurobiol. 32, 284–295 (2006).
Minguez-Castellanos, A. et al. Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology 68, 2012–2018 (2007).
Fujishiro, H. et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson's disease. Mov. Disord. 23, 1085–1092 (2008).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 (2003).
Lees, A. J. The Parkinson chimera. Neurology 72 (7 Suppl.), S2–S11 (2009).
Dexter, D. T. et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann. Neurol. 35, 38–44 (1994).
DelleDonne, A. et al. Incidental Lewy body disease and preclinical Parkinson disease. Arch. Neurol. 65, 1074–1080 (2008).
Greffard, S. et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol. 63, 584–588 (2006).
Bezard, E. et el. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J. Neurosci. 21, 6853–6861 (2001).
Schapira, A. H. et al. Novel pharmacological targets for the treatment of Parkinson's disease. Nat. Rev. Drug Discov. 5, 845–854 (2006).
Markham, C. H. & Diamond S. G. Evidence to support early levodopa therapy in Parkinson disease. Neurology 31, 125–131 (1981).
Grosset, D. et al. A multicentre longitudinal observational study of changes in self reported health status in people with Parkinson's disease left untreated at diagnosis. J. Neurol. Neurosurg. Psychiatry 78, 465–469 (2007).
Schapira, A. H. & Obeso, J. Timing of treatment initiation in Parkinson's disease: a need for reappraisal? Ann. Neurol. 59, 559–562 (2006).
Brotchie, J. & Fitzer-Attas, C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology 72 (7 Suppl.), S32–S38 (2009).
Olanow, C. W. et al. A double-blind, delayed-start trial of rasagiline in Parkinson's disease. N. Engl. J. Med. 361, 1268–1278 (2009).
Schapira, A. H. Molecular and clinical pathways to neuroprotection of dopaminergic drugs in Parkinson disease. Neurology 72 (7 Suppl.), S44–S50 (2009).
Schapira, A. H. Science, medicine, and the future: Parkinson's disease. BMJ 318, 311–314 (1999).
Hart, R. G., Pearce, L. A., Ravina, B. M, Yaltho, T. C. & Marler, J. R. Neuroprotection trials in Parkinson's disease: systematic review. Mov. Disord. 24, 647–654 (2009).
Olanow, C. W., Kieburtz, K. & Schapira A. H. Why have we failed to achieve neuroprotection in Parkinson's disease? Ann. Neurol. 64 (Suppl. 2), S101–S110 (2008).
Rascol, O. “Disease-modification” trials in Parkinson disease: target populations, endpoints and study design. Neurology 72 (7 Suppl.), S51–S58 (2009).
Olanow, C. W. et al. TCH346 as a neuroprotective drug in Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 5, 1013–1020 (2006).
Lang, A. E. When and how should treatment be started in Parkinson disease? Neurology 72 (7 Suppl.), S39–S43 (2009).
Goetz, C. G., Poewe W., Rascol, O. & Sampaio C. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004. Mov. Disord. 20, 523–539 (2005).
Pahwa, R. et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66, 983–995 (2006).
Schapira, A. H. Treatment options in the modern management of Parkinson disease. Arch. Neurol. 64, 1083–1088 (2007).
Schapira, A. H., Emre, M., Jenner, P. & Poewe, W. Levodopa in the treatment of Parkinson's disease. Eur. J. Neurol. 16, 982–989 (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A. H. V. Schapira has acted as a consultant for and received honoraria from Boehringer, GlaxoSmithKline, Novartis-Orion and Teva-Lundbeck, and has received research support from Boehringer and Teva-Lundbeck. E. Tolosa declares no competing interests.
Rights and permissions
About this article
Cite this article
Schapira, A., Tolosa, E. Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat Rev Neurol 6, 309–317 (2010). https://doi.org/10.1038/nrneurol.2010.52
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.52
This article is cited by
-
Shrinkage of olfactory amygdala connotes cognitive impairment in patients with Parkinson’s disease
Cognitive Neurodynamics (2023)
-
RBD and hyposmia in Moroccan patients with a synucleinopathy: prevalence and the timing of occurrence in a large cohort
Acta Neurologica Belgica (2023)
-
Differential serum microRNAs in premotor LRRK2 G2019S carriers from Parkinson’s disease
npj Parkinson's Disease (2023)
-
The use of fibroblasts as a valuable strategy for studying mitochondrial impairment in neurological disorders
Translational Neurodegeneration (2022)
-
Gaucher disease – more than just a rare lipid storage disease
Journal of Molecular Medicine (2022)