Key Points
-
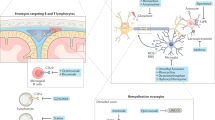
Neuronal and axonal degeneration in multiple sclerosis (MS) is a slow process initiated by acute lymphocytic inflammation, and subsequently driven by chronically smouldering, diffuse parenchymal myeloid and meningeal lymphocytic inflammation
-
Oxidative stress, mitochondrial injury and subsequent ion channel dysfunction secondary to chronic inflammation seem to have a constant impact on neurons and axons, leading to their demise during progressive MS
-
Several ion channels show compensatory changes in response to the inflammatory stimulus by altering their relative distribution in the neuron—a process that eventually becomes maladaptive and perpetuates neuroaxonal injury
-
Several neuroprotective pathways have been identified in MS, but these pathways become overridden, resulting in neuronal degeneration that is probably mediated by the initiation of apoptosis and Wallerian degeneration
-
The balance between continuous inflammatory stressors and intrinsic buffering mechanisms depends partly on age, sex and genetic factors, which eventually determine the clinical course of MS
-
In an animal model of MS, few molecular targets with proven neuroprotective properties that are separable from their impact on inflammatory responses have been identified; these molecules include CyPD, ASIC1 and TRPM4
Abstract
Multiple sclerosis (MS) is the most frequent chronic inflammatory disease of the CNS, and imposes major burdens on young lives. Great progress has been made in understanding and moderating the acute inflammatory components of MS, but the pathophysiological mechanisms of the concomitant neurodegeneration—which causes irreversible disability—are still not understood. Chronic inflammatory processes that continuously disturb neuroaxonal homeostasis drive neurodegeneration, so the clinical outcome probably depends on the balance of stressor load (inflammation) and any remaining capacity for neuronal self-protection. Hence, suitable drugs that promote the latter state are sorely needed. With the aim of identifying potential novel therapeutic targets in MS, we review research on the pathological mechanisms of neuroaxonal dysfunction and injury, such as altered ion channel activity, and the endogenous neuroprotective pathways that counteract oxidative stress and mitochondrial dysfunction. We focus on mechanisms inherent to neurons and their axons, which are separable from those acting on inflammatory responses and might, therefore, represent bona fide neuroprotective drug targets with the capability to halt MS progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Weiner, H. L. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 255 (Suppl. 1), 3–11 (2008).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Frischer, J. M. et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132, 1175–1189 (2009).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000).
Kutzelnigg, A. et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712 (2005).
DeLuca, G. C., Ebers, G. C. & Esiri, M. M. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain 127, 1009–1018 (2004).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
DeLuca, G. C., Williams, K., Evangelou, N., Ebers, G. C. & Esiri, M. M. The contribution of demyelination to axonal loss in multiple sclerosis. Brain 129, 1507–1516 (2006).
Scalfari, A., Neuhaus, A., Daumer, M., Muraro, P. A. & Ebers, G. C. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 85, 67–75 (2014).
Confavreux, C., Vukusic, S., Moreau, T. & Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 343, 1430–1438 (2000).
Scalfari, A. et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 133, 1914–1929 (2010).
Scalfari, A. et al. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 70, 214–222 (2013).
Kappos, L. et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 8, 987–997 (2009).
Shirani, A. et al. Association between use of interferon beta and progression of disability in patients with relapsing–remitting multiple sclerosis. JAMA 308, 247–256 (2012).
Haghikia, A., Hohlfeld, R., Gold, R. & Fugger, L. Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol. Med. 19, 309–319 (2013).
Barkhof, F., Calabresi, P. A., Miller, D. H. & Reingold, S. C. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat. Rev. Neurol. 5, 256–266 (2009).
Chard, D. T. et al. Brain atrophy in clinically early relapsing–remitting multiple sclerosis. Brain 125, 327–337 (2002).
Miller, D. H., Barkhof, F., Frank, J. A., Parker, G. J. & Thompson, A. J. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125, 1676–1695 (2002).
Petzold, A. et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 9, 921–932 (2010).
Kuhle, J. et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 76, 1206–1213 (2011).
Gunnarsson, M. et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 69, 83–89 (2011).
Popescu, B. F. & Lucchinetti, C. F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 7, 185–217 (2012).
Bjartmar, C., Kidd, G., Mörk, S., Rudick, R. & Trapp, B. D. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann. Neurol. 48, 893–901 (2000).
Dziedzic, T. et al. Wallerian degeneration: a major component of early axonal pathology in multiple sclerosis. Brain Pathol. 20, 976–985 (2010).
Peterson, J. W., Bö, L., Mörk, S., Chang, A. & Trapp, B. D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50, 389–400 (2001).
Vercellino, M. et al. Grey matter pathology in multiple sclerosis. J. Neuropathol. Exp. Neurol. 64, 1101–1107 (2005).
Lucchinetti, C. F. et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197 (2011).
Kutzelnigg, A. et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 17, 38–44 (2007).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Howell, O. W. et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–2771 (2011).
DeLuca, G. C. et al. Casting light on multiple sclerosis heterogeneity: the role of HLA-DRB1 on spinal cord pathology. Brain 136, 1025–1034 (2013).
The International Multiple Sclerosis Genetics Consortium. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357, 851–862 (2007).
Baranzini, S. E. et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum. Mol. Genet. 18, 767–778 (2009).
Brynedal, B. et al. MGAT5 alters the severity of multiple sclerosis. J. Neuroimmunol. 220, 120–124 (2010).
International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes Immun. 12, 615–625 (2011).
Baranzini, S. E. et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 133, 2603–2611 (2010).
Friese, M. A. et al. The value of animal models for drug development in multiple sclerosis. Brain 129, 1940–1952 (2006).
Haider, L. et al. Oxidative damage in multiple sclerosis lesions. Brain 134, 1914–1924 (2011).
Zeis, T. et al. Molecular changes in white matter adjacent to an active demyelinating lesion in early multiple sclerosis. Brain Pathol. 19, 459–466 (2009).
Nikic, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Fischer, M. T. et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 136, 1799–1815 (2013).
Gray, E., Thomas, T. L., Betmouni, S., Scolding, N. & Love, S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 18, 86–95 (2008).
Fischer, M. T. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135, 886–899 (2012).
Li, S., Vana, A. C., Ribeiro, R. & Zhang, Y. Distinct role of nitric oxide and peroxynitrite in mediating oligodendrocyte toxicity in culture and in experimental autoimmune encephalomyelitis. Neuroscience 184, 107–119 (2011).
Smith, K. J., Kapoor, R., Hall, S. M. & Davies, M. Electrically active axons degenerate when exposed to nitric oxide. Ann. Neurol. 49, 470–476 (2001).
Hametner, S. et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 74, 846–861 (2013).
Rathore, K. I. et al. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J. Neurosci. 28, 12736–12747 (2008).
Craelius, W., Migdal, M. W., Luessenhop, C. P., Sugar, A. & Mihalakis, I. Iron deposits surrounding multiple sclerosis plaques. Arch. Pathol. Lab. Med. 106, 397–399 (1982).
Bagnato, F. et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134, 3602–3615 (2011).
Lopes, K. O., Sparks, D. L. & Streit, W. J. Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia 56, 1048–1060 (2008).
Lassmann, H., van Horssen, J. & Mahad, D. Progressive multiple sclerosis: pathology and pathogenesis. Nat. Rev. Neurol. 8, 647–656 (2012).
Nathoo, N. et al. Susceptibility-weighted imaging in the experimental autoimmune encephalomyelitis model of multiple sclerosis indicates elevated deoxyhemoglobin, iron deposition and demyelination. Mult. Scler. 19, 721–731 (2013).
Kensler, T. W., Wakabayashi, N. & Biswal, S. Cell survival responses to environmental stresses via the Keap1–Nrf2–ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89–116 (2007).
van Horssen, J. et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic. Biol. Med. 45, 1729–1737 (2008).
Chora, A. A. et al. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Invest. 117, 438–447 (2007).
Pennisi, G. et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 82, 1490–1499 (2011).
Johnson, D. A., Amirahmadi, S., Ward, C., Fabry, Z. & Johnson, J. A. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol. Sci. 114, 237–246 (2010).
Linker, R. A. et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692 (2011).
Ghoreschi, K. et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 208, 2291–2303 (2011).
Schilling, S., Goelz, S., Linker, R., Luehder, F. & Gold, R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin. Exp. Immunol. 145, 101–107 (2006).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Trapp, B. D. & Stys, P. K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8, 280–291 (2009).
Wallace, D. C., Fan, W. & Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 5, 297–348 (2010).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Echaniz-Laguna, A. et al. POLG1 variations presenting as multiple sclerosis. Arch. Neurol. 67, 1140–1143 (2010).
Kim, J. Y. et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat. Neurosci. 13, 180–189 (2010).
Shindler, K. S. et al. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 30, 328–339 (2010).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 (2012).
Forte, M. et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl Acad. Sci. USA 104, 7558–7563 (2007).
Friese, M. A. et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13, 1483–1489 (2007).
Aboul-Enein, F. et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J. Neuropathol. Exp. Neurol. 62, 25–33 (2003).
Graumann, U., Reynolds, R., Steck, A. J. & Schaeren-Wiemers, N. Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol. 13, 554–573 (2003).
Howarth, C., Gleeson, P. & Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 32, 1222–1232 (2012).
Paling, D. et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain 136, 2305–2317 (2013).
Young, E. A. et al. Imaging correlates of decreased axonal Na+/K+ ATPase in chronic multiple sclerosis lesions. Ann. Neurol. 63, 428–435 (2008).
Craner, M. J. et al. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl Acad. Sci. USA 101, 8168–8173 (2004).
Alle, H., Roth, A. & Geiger, J. R. Energy-efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408 (2009).
Carter, B. C. & Bean, B. P. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron 64, 898–909 (2009).
Hodgkin, A. The optimum density of sodium channels in an unmyelinated nerve. Philos. Trans. R. Soc. Lond. B Biol. Sci. 270, 297–300 (1975).
Craner, M. J., Hains, B. C., Lo, A. C., Black, J. A. & Waxman, S. G. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127, 294–303 (2004).
O'Malley, H. A., Shreiner, A. B., Chen, G. H., Huffnagle, G. B. & Isom, L. L. Loss of Na+ channel β2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol. Cell. Neurosci. 40, 143–155 (2009).
Bechtold, D. A., Kapoor, R. & Smith, K. J. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann. Neurol. 55, 607–616 (2004).
Morsali, D. et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain 136, 1067–1082 (2013).
Black, J. A., Liu, S., Carrithers, M., Carrithers, L. M. & Waxman, S. G. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann. Neurol. 62, 21–33 (2007).
Lo, A. C., Saab, C. Y., Black, J. A. & Waxman, S. G. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J. Neurophysiol. 90, 3566–3571 (2003).
Bechtold, D. A. et al. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J. Neurol. 253, 1542–1551 (2006).
Craner, M. J. et al. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia 49, 220–229 (2005).
Kapoor, R. et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 9, 681–688 (2010).
Shields, S. D. et al. A channelopathy contributes to cerebellar dysfunction in a model of multiple sclerosis. Ann. Neurol. 71, 186–194 (2012).
Moran, M. M., McAlexander, M. A., Biro, T. & Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10, 601–620 (2011).
Guinamard, R., Demion, M. & Launay, P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 25, 155–164 (2010).
Schattling, B. et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 18, 1805–1811 (2012).
Dunn, J. & Blight, A. Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis. Curr. Med. Res. Opin. 27, 1415–1423 (2011).
Jukkola, P. I., Lovett-Racke, A. E., Zamvil, S. S. & Gu, C. K+ channel alterations in the progression of experimental autoimmune encephalomyelitis. Neurobiol. Dis. 47, 280–293 (2012).
Beraud, E. et al. Block of neural Kv1.1 potassium channels for neuroinflammatory disease therapy. Ann. Neurol. 60, 586–596 (2006).
Mathie, A. & Veale, E. L. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr. Opin. Investig. Drugs 8, 555–562 (2007).
Bittner, S. et al. TASK1 modulates inflammation and neurodegeneration in autoimmune inflammation of the central nervous system. Brain 132, 2501–2516 (2009).
Bittner, S. et al. The TASK1 channel inhibitor A293 shows efficacy in a mouse model of multiple sclerosis. Exp. Neurol. 238, 149–155 (2012).
Brand-Schieber, E. & Werner, P. Calcium channel blockers ameliorate disease in a mouse model of multiple sclerosis. Exp. Neurol. 189, 5–9 (2004).
Waxman, S. G. & Ritchie, J. M. Molecular dissection of the myelinated axon. Ann. Neurol. 33, 121–136 (1993).
Kornek, B. et al. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 124, 1114–1124 (2001).
Gadjanski, I. et al. Role of N-type voltage-dependent calcium channels in autoimmune optic neuritis. Ann. Neurol. 66, 81–93 (2009).
Stirling, D. P. & Stys, P. K. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends Mol. Med. 16, 160–170 (2010).
Hardingham, G. E. & Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 (2010).
Mahad, D. J. et al. Mitochondrial changes within axons in multiple sclerosis. Brain 132, 1161–1174 (2009).
Grasselli, G. et al. Abnormal NMDA receptor function exacerbates experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 168, 502–517 (2013).
Sarchielli, P., Greco, L., Floridi, A. & Gallai, V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch. Neurol. 60, 1082–1088 (2003).
Piani, D., Frei, K., Do, K. Q., Cuenod, M. & Fontana, A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci. Lett. 133, 159–162 (1991).
Newcombe, J. et al. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 18, 52–61 (2008).
Vercellino, M. et al. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J. Neuropathol. Exp. Neurol. 66, 732–739 (2007).
Hardin-Pouzet, H. et al. Glutamate metabolism is down-regulated in astrocytes during experimental allergic encephalomyelitis. Glia 20, 79–85 (1997).
Ohgoh, M. et al. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 125, 170–178 (2002).
Pitt, D., Werner, P. & Raine, C. S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6, 67–70 (2000).
Smith, T., Groom, A., Zhu, B. & Turski, L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 6, 62–66 (2000).
Kanwar, J. R., Kanwar, R. K. & Krissansen, G. W. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain 127, 1313–1331 (2004).
Paul, C. & Bolton, C. Modulation of blood–brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-D-aspartate receptor antagonist memantine. J. Pharmacol. Exp. Ther. 302, 50–57 (2002).
Wallstrom, E. et al. Memantine abrogates neurological deficits, but not CNS inflammation, in Lewis rat experimental autoimmune encephalomyelitis. J. Neurol. Sci. 137, 89–96 (1996).
Centonze, D. et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci. 29, 3442–3452 (2009).
Basso, A. S. et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J. Clin. Invest. 118, 1532–1543 (2008).
Wemmie, J. A., Taugher, R. J. & Kreple, C. J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 14, 461–471 (2013).
Yermolaieva, O., Leonard, A. S., Schnizler, M. K., Abboud, F. M. & Welsh, M. J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl Acad. Sci. USA 101, 6752–6757 (2004).
Vergo, S. et al. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain 134, 571–584 (2011).
Arun, T. et al. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain 136, 106–115 (2013).
Hassen, G. W., Feliberti, J., Kesner, L., Stracher, A. & Mokhtarian, F. Prevention of axonal injury using calpain inhibitor in chronic progressive experimental autoimmune encephalomyelitis. Brain Res. 1236, 206–215 (2008).
Shields, D. C., Tyor, W. R., Deibler, G. E., Hogan, E. L. & Banik, N. L. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc. Natl Acad. Sci. USA 95, 5768–5772 (1998).
Guyton, M. K. et al. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J. Neurosci. Res. 81, 53–61 (2005).
Offen, D. et al. Mice overexpressing Bcl-2 in their neurons are resistant to myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE). J. Mol. Neurosci. 15, 167–176 (2000).
Aktas, O. et al. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 46, 421–432 (2005).
Chitnis, T. et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am. J. Pathol. 170, 1695–1712 (2007).
Saxena, S. & Caroni, P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48 (2011).
Kemp, J. A. & McKernan, R. M. NMDA receptor pathways as drug targets. Nat. Neurosci. 5 (Suppl.), 1039–1042 (2002).
Stranahan, A. M. & Mattson, M. P. Recruiting adaptive cellular stress responses for successful brain ageing. Nat. Rev. Neurosci. 13, 209–216 (2012).
Prakash, R. S., Snook, E. M., Motl, R. W. & Kramer, A. F. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 1341, 41–51 (2010).
Briken, S. et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Mult. Scler. http://dx.doi.org/10.1177/1352458513507358.
Rossi, S. et al. Exercise attenuates the clinical, synaptic and dendritic abnormalities of experimental autoimmune encephalomyelitis. Neurobiol. Dis. 36, 51–59 (2009).
Safdar, A. et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl Acad. Sci. USA 108, 4135–4140 (2011).
Maresz, K. et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 13, 492–497 (2007).
Kaneko, S. et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 (2006).
Dutta, R. et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 59, 478–489 (2006).
Campbell, G. R. et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 69, 481–492 (2011).
Mahad, D., Ziabreva, I., Lassmann, H. & Turnbull, D. Mitochondrial defects in acute multiple sclerosis lesions. Brain 131, 1722–1735 (2008).
Zambonin, J. L. et al. Increased mitochondrial content in remyelinated axons: implications for multiple sclerosis. Brain 134, 1901–1913 (2011).
Linker, R. A. et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat. Med. 8, 620–624 (2002).
Linker, R. A. et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain 133, 2248–2263 (2010).
Lu, B., Nagappan, G., Guan, X., Nathan, P. J. & Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 401–416 (2013).
Petryshen, T. L. et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry 15, 810–815 (2010).
Zivadinov, R. et al. Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum. Mol. Genet. 16, 2659–2668 (2007).
Ramasamy, D. P. et al. Effect of Met66 allele of the BDNF rs6265 SNP on regional gray matter volumes in patients with multiple sclerosis: a voxel-based morphometry study. Pathophysiology 18, 53–60 (2011).
Nagahara, A. H. & Tuszynski, M. H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 10, 209–219 (2011).
Stadelmann, C. et al. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain 125, 75–85 (2002).
Kerschensteiner, M. et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 189, 865–870 (1999).
Lee, D. H. et al. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 123, 247–258 (2012).
Colombo, E. et al. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J. Exp. Med. 209, 521–535 (2012).
Jones, J. L. et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain 133, 2232–2247 (2010).
Thone, J. et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am. J. Pathol. 180, 267–274 (2012).
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Pryce, G. et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126, 2191–2202 (2003).
Rossi, S. et al. Cannabinoid CB1 receptors regulate neuronal TNF-α effects in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 25, 1242–1248 (2011).
Croxford, J. L. et al. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J. Neuroimmunol. 193, 120–129 (2008).
Zajicek, J. et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 12, 857–865 (2013).
Broxmeyer, H. E. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 210, 205–208 (2013).
Li, W. et al. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann. Neurol. 56, 767–777 (2004).
Suhs, K. W. et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann. Neurol. 72, 199–210 (2012).
Acknowledgements
M.A.F. is supported by the Deutsche Forschungsgemeinschaft Emmy Noether-Programme (FR1720/3-1), Gemeinnützige Hertie-Stiftung (1.01.1/11/003 and P1130075), Werner Otto Stiftung (1/81), Forschungs- und Wissenschaftsstiftung Hamburg, Boehringer Ingelheim Stiftung Exploration Grant and BMBF Biopharma (NEU2 programme). L.F. is supported by the Wellcome Trust, the Medical Research Council, the Lundbeck Foundation and the Naomi Bransom Foundation.
Author information
Authors and Affiliations
Contributions
M.A.F. researched most of the data and drafted the article with substantial contributions from B.S. and L.F. All authors contributed to discussion of the content, reviewing, and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Pharmacological ion channel modulation in EAE (DOC 101 kb)
Rights and permissions
About this article
Cite this article
Friese, M., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol 10, 225–238 (2014). https://doi.org/10.1038/nrneurol.2014.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.37
This article is cited by
-
The aging mouse CNS is protected by an autophagy-dependent microglia population promoted by IL-34
Nature Communications (2024)
-
Spatial correspondence among regional gene expressions and gray matter volume loss in multiple sclerosis
Molecular Psychiatry (2024)
-
Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice
Molecular Neurobiology (2024)
-
Microglial aryl hydrocarbon receptor enhances phagocytic function via SYK and promotes remyelination in the cuprizone mouse model of demyelination
Journal of Neuroinflammation (2023)
-
Correspondence among gray matter atrophy and atlas-based neurotransmitter maps is clinically relevant in multiple sclerosis
Molecular Psychiatry (2023)