Abstract
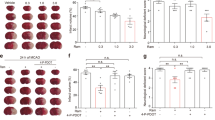
Neuroprotectant strategies that have worked in rodent models of stroke have failed to provide protection in clinical trials. Here we show that the opposite circadian cycles in nocturnal rodents versus diurnal humans1,2 may contribute to this failure in translation. We tested three independent neuroprotective approaches—normobaric hyperoxia, the free radical scavenger α-phenyl-butyl-tert-nitrone (αPBN), and the N-methyl-d-aspartic acid (NMDA) antagonist MK801—in mouse and rat models of focal cerebral ischaemia. All three treatments reduced infarction in day-time (inactive phase) rodent models of stroke, but not in night-time (active phase) rodent models of stroke, which match the phase (active, day-time) during which most strokes occur in clinical trials. Laser-speckle imaging showed that the penumbra of cerebral ischaemia was narrower in the active-phase mouse model than in the inactive-phase model. The smaller penumbra was associated with a lower density of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive dying cells and reduced infarct growth from 12 to 72 h. When we induced circadian-like cycles in primary mouse neurons, deprivation of oxygen and glucose triggered a smaller release of glutamate and reactive oxygen species, as well as lower activation of apoptotic and necroptotic mediators, in ‘active-phase’ than in ‘inactive-phase’ rodent neurons. αPBN and MK801 reduced neuronal death only in ‘inactive-phase’ neurons. These findings suggest that the influence of circadian rhythm on neuroprotection must be considered for translational studies in stroke and central nervous system diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Source Data associated with Figures and Extended Data are available online.
Change history
13 June 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41586-020-2427-1
References
Logan, R. W. & McClung, C. A. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65 (2019).
Cederroth, C. R. et al. Medicine in the fourth dimension. Cell Metab. 30, 238–250 (2019).
VISTA Database http://www.virtualtrialsarchives.org/vista/ (2019).
Ding, J. et al. The effect of normobaric oxygen in patients with acute stroke: a systematic review and meta-analysis. Neurol. Res. 40, 433–444 (2018).
Green, A. R., Ashwood, T., Odergren, T. & Jackson, D. M. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharmacol. Ther. 100, 195–214 (2003).
Schurr, A. Neuroprotection against ischemic/hypoxic brain damage: blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr. Drug Targets 5, 603–618 (2004).
Neuhaus, A. A., Couch, Y., Hadley, G. & Buchan, A. M. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 140, 2079–2092 (2017).
Campbell, B. C., Meretoja, A., Donnan, G. A. & Davis, S. M. Twenty-year history of the evolution of stroke thrombolysis with intravenous alteplase to reduce long-term disability. Stroke 46, 2341–2346 (2015).
Campbell, B. C. V. et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 14, 846–854 (2015).
Pritchett, D. & Reddy, A. B. Circadian clocks in the hematologic system. J. Biol. Rhythms 30, 374–388 (2015).
Schulz, J. B. et al. Facilitation of postischemic reperfusion with α-PBN: assessment using NMR and Doppler flow techniques. Am. J. Physiol. 272, H1986–H1995 (1997).
Buchan, A. & Pulsinelli, W. A. Hypothermia but not the N-methyl-d-aspartate antagonist, MK-801, attenuates neuronal damage in gerbils subjected to transient global ischemia. J. Neurosci. 10, 311–316 (1990).
Lo, E. H. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 14, 497–500 (2008).
Donnan, G. A., Baron, J. C., Ma, H. & Davis, S. M. Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol. 8, 261–269 (2009).
Heiss, W. D. Experimental evidence of ischemic thresholds and functional recovery. Stroke 23, 1668–1672 (1992).
Hossmann, K. A. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 36, 557–565 (1994).
Mure, L. S. et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318 (2018).
Chen, C. Y. et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl Acad. Sci. USA 113, 206–211 (2016).
Balsalobre, A. et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 (2000).
Fan, J., Dawson, T. M. & Dawson, V. L. Cell death mechanisms of neurodegeneration. Adv. Neurobiol. 15, 403–425 (2017).
Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 (1999).
Marshall, J. W., Duffin, K. J., Green, A. R. & Ridley, R. M. NXY-059, a free radical-trapping agent, substantially lessens the functional disability resulting from cerebral ischemia in a primate species. Stroke 32, 190–198 (2001).
Marshall, J. W., Cummings, R. M., Bowes, L. J., Ridley, R. M. & Green, A. R. Functional and histological evidence for the protective effect of NXY-059 in a primate model of stroke when given 4 hours after occlusion. Stroke 34, 2228–2233 (2003).
Paschos, G. K. & FitzGerald, G. A. Circadian clocks and vascular function. Circ. Res. 106, 833–841 (2010).
Durgan, D. J., Crossland, R. F. & Bryan, R. M. Jr. The rat cerebral vasculature exhibits time-of-day-dependent oscillations in circadian clock genes and vascular function that are attenuated following obstructive sleep apnea. J. Cereb. Blood Flow Metab. 37, 2806–2819 (2017).
Musiek, E. S. et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400 (2013).
Kobayashi, Y., Ye, Z. & Hensch, T. K. Clock genes control cortical critical period timing. Neuron 86, 264–275 (2015).
Banks, W. A. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
Lo, E. H., Dalkara, T. & Moskowitz, M. A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4, 399–415 (2003).
Shi, L. et al. A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J. Cereb. Blood Flow Metab. 38, 2073–2091 (2018).
Poli, S. & Veltkamp, R. Oxygen therapy in acute ischemic stroke—experimental efficacy and molecular mechanisms. Curr. Mol. Med. 9, 227–241 (2009).
Buchan, A. M., Slivka, A. & Xue, D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res. 574, 171–177 (1992).
Cao, X. & Phillis, J. W. α-Phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Res. 644, 267–272 (1994).
Pschorn, U. & Carter, A. J. The influence of repeated doses, route and time of administration on the neuroprotective effects of BIII 277 CL in a rat model of focal cerebral ischemia. J. Stroke Cerebrovasc. Dis. 6, 93–99 (1996).
Folbergrová, J., Zhao, Q., Katsura, K. & Siesjö, B. K. N-tert-butyl-alpha-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc. Natl Acad. Sci. USA 92, 5057–5061 (1995).
Reimherr, F. W., Wood, D. R. & Wender, P. H. The use of MK-801, a novel sympathomimetic, in adults with attention deficit disorder, residual type. Psychopharmacol. Bull. 22, 237–242 (1986).
Shuaib, A. et al. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 357, 562–571 (2007).
Vinall, P. E., Kramer, M. S., Heinel, L. A. & Rosenwasser, R. H. Temporal changes in sensitivity of rats to cerebral ischemic insult. J. Neurosurg. 93, 82–89 (2000).
Tischkau, S. A., Cohen, J. A., Stark, J. T., Gross, D. R. & Bottum, K. M. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Exp. Neurol. 208, 314–322 (2007).
Beker, M. C. et al. Time-of-day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase Akt. Mol. Neurobiol. 55, 2565–2576 (2018).
Ali, K., Cheek, E., Sills, S., Crome, P. & Roffe, C. Day-night differences in oxygen saturation and the frequency of desaturations in the first 24 hours in patients with acute stroke. J. Stroke Cerebrovasc. Dis. 16, 239–244 (2007).
Kim, B. J. et al. Ischemic stroke during sleep: its association with worse early functional outcome. Stroke 42, 1901–1906 (2011).
Karmarkar, S. W. & Tischkau, S. A. Influences of the circadian clock on neuronal susceptibility to excitotoxicity. Front. Physiol. 4, 313 (2013).
Wang, T. A. et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 337, 839–842 (2012).
Ghorbel, M. T., Coulson, J. M. & Murphy, D. Cross-talk between hypoxic and circadian pathways: cooperative roles for hypoxia-inducible factor 1α and CLOCK in transcriptional activation of the vasopressin gene. Mol. Cell. Neurosci. 22, 396–404 (2003).
Lorenzano, S. et al. Early molecular oxidative stress biomarkers of ischemic penumbra in acute stroke. Neurology 93, e1288–e1298 (2019).
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (K99MH120053 (I.Ş.), R01NS091230 and R01MH111359 (S.S.)), the Rappaport Foundation (E.H.L.), the Chinese Ministry of Education (X.J.), and the National Research Foundation of Korea (J.-H.P.). The authors thank J. Lipton, M. Ning and W. Deng for discussions, Y. Sun and all team members of the MGH 149-8 animal facility for help with light schedule switching, and M. Ali and K. R. Lees for generous assistance and expert analysis of the VISTA database.
Author information
Authors and Affiliations
Contributions
Performed experiments and/or analysed data: (E.E., W.L., E.T.M., J.-H.P., I. Ş., S.G., J.S., J. Lan, J. Lee, K.H.); designed experiments (E.E., W.L., E.T.M., J.-H.P., I. Ş., S.S., E.H.L.); wrote and/or revised manuscript (E.E., W.L., E.T.M., K.H., X.J., E.H.L.); funding and support (J.-H.P., S.S., X.J., E.H.L.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks John Hogenesch, Costantino Iadecola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Clot lysis.
Rates of tissue-plasminogen activator-induced clot lysis were not significantly different in blood drawn from day-time (ZT3–9, n = 5) or night-time (ZT15–21, n = 8) male C57BL/6 mice. Mean ± s.e.m., repeated-measures ANOVA; P = 0.80.
Extended Data Fig. 2 Effects of αPBN on reperfusion.
Laser Doppler flowmetry showed that αPBN (100 mg kg−1) did not affect reperfusion profiles after 60-min transient focal ischaemia in ZT3–9 or ZT15–21 male C57BL/6 mice. Mean ± s.e.m., n = 4 per group, repeated-measures ANOVA; P = 0.87.
Extended Data Fig. 3 MK801 did not significantly affect body temperature after permanent focal cerebral ischaemia.
White bars, ZT3–9; grey bars, ZT15–21; male C57BL/6 mice. Mean ± s.e.m., n = 4 per group, repeated-measures ANOVA; P = 0.97.
Extended Data Fig. 4 Physiological parameters for laser speckle imaging experiments in Fig. 2.
n = 4 mice per group; mean ± s.e.m., two-tailed t-test.
Extended Data Fig. 5 Quantification of blood flow.
Speckle imaging data were used to quantify blood flow in terms of absolute blood flow (ml per 100 g per min). Blood flow histograms show the presence of cerebral ischaemia in the ipsilateral hemispheres (top). Thresholded areas between 25 and 55 ml per 100 g per min (see Methods) were significantly smaller in ZT17–19 than in ZT5–7 C57BL/6 male mice (bottom; n = 4 per group, two-tailed t-test).
Extended Data Fig. 6 Circadian gene expression and patterns in different species.
Top, comparison of circadian gene patterns between mice, rats, nonhuman primates and humans. Bottom, expression of selected circadian genes in C57BL/6 male mouse and Sprague–Dawley male rat somatosensory cortex (n = 4 per group).
Extended Data Fig. 7 Blood cortisol levels.
No significant differences were detected in blood cortisol levels of ZT3–9 versus ZT15–21 C57BL/6 male mice subjected to similar handling procedures as cerebral ischaemia mice. Mean ± s.e.m., n = 5 mice per group, two-tailed t-test.
Supplementary information
Supplementary Figure
This file contains the uncropped gel images.
Source data
Rights and permissions
About this article
Cite this article
Esposito, E., Li, W., T. Mandeville, E. et al. Potential circadian effects on translational failure for neuroprotection. Nature 582, 395–398 (2020). https://doi.org/10.1038/s41586-020-2348-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2348-z
This article is cited by
-
Glymphatic and lymphatic communication with systemic responses during physiological and pathological conditions in the central nervous system
Communications Biology (2024)
-
Circadian rhythm in cardiovascular diseases: a bibliometric analysis of the past, present, and future
European Journal of Medical Research (2023)
-
Circadian clock regulator Bmal1 gates axon regeneration via Tet3 epigenetics in mouse sensory neurons
Nature Communications (2023)
-
A New Paradigm for Neuroprotection Clinical Trials for Acute Ischemic Stroke
Translational Stroke Research (2023)
-
Dual Antioxidant DH-217 Mitigated Cerebral Ischemia–Reperfusion Injury by Targeting IKKβ/Nrf2/HO-1 Signal Axis
Neurochemical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.