-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Confavreux, Sandra Vukusic, Age at disability milestones in multiple sclerosis, Brain, Volume 129, Issue 3, March 2006, Pages 595–605, https://doi.org/10.1093/brain/awh714
Close - Share Icon Share
Abstract
Many efforts have been devoted to the description of the prognosis of multiple sclerosis and its possible influential factors in terms of time to reach disability milestones. By contrast, the age at which patients with multiple sclerosis reach these milestones has not yet stirred much interest. We have tested the hypothesis whether the prognosis of multiple sclerosis depends on the current age of patients and the initial course of the disease. We have assessed disease onset and course, and assignment of scores of irreversible disability in 1844 patients with multiple sclerosis. We have used three scores on the Kurtzke Disability Status Scale as benchmarks of disability accumulation: DSS 4 (limited walking but without aid), DSS 6 (walking with unilateral aid) and DSS 7 (wheelchair-bound). We used Kaplan–Meier analyses to estimate the age of the patients at assignment of disability milestones. The possible influence of the initial course of multiple sclerosis and of other clinical variables early assessable in the disease on these outcome measures was also studied, using the Kaplan–Meier curves for univariate analyses and Cox models for multivariate analyses. For the 1844 patients, median ages at time of assignment of irreversible disability were 44.3 years (95% CI 43.3–45.2) for a score of DSS 4, 54.7 years (95% CI 53.5–55.8) for DSS 6 and 63.1 years (95% CI 61.0–65.1) for DSS 7. These results were essentially similar whether the initial course of multiple sclerosis was exacerbating-remitting or progressive, and whatever the initial symptomatology. Females reached disability milestones at an older age than males. The most influential clinical factor was age at clinical onset of multiple sclerosis: the younger the onset, the younger the age at assignment of disability milestones. Therefore, prognosis in multiple sclerosis appears, at least to some extent, as age-dependent and not substantially affected by the initial course, be it exacerbating-remitting or progressive. Aside acute focal recurrent inflammation and diffuse chronic neurodegeneration, accelerated ageing-related mechanisms may operate in the central nervous system of multiple sclerosis patients.
Introduction
For decades, efforts have concentrated on the description of the prognosis of multiple sclerosis and its possible influential factors, providing quite a number of series which are, in many respects, representative of the disease in an essentially untreated population. Many of them are based upon more or less complete prevalence material with a cross-sectional and, in some cases, some longitudinal assessment (Müller, 1949, 1951; McAlpine and Compston, 1952; Leibowitz et al., 1964a, b; Panelius, 1969; Leibowitz and Alter, 1970, 1973; Poser, 1978; Poser et al., 1982; Clark et al., 1982; Detels et al., 1982; Visscher et al., 1984; Minderhoud et al., 1988; Phadke, 1990; Miller et al., 1992; Riise et al., 1992; Trojano et al., 1995; Kantarci et al., 1998; Amato and Ponziani, 2000; Myhr et al., 2001), whereas four series, that of the United States Army Veterans World War II (Kurtzke et al., 1968, 1970, 1973, 1977), Lyon, France (Confavreux, 1977; Confavreux et al., 1980, 2000, 2003), Gothenburg, Sweden (Broman et al., 1981; Runmarker and Andersen, 1993; Eriksson et al., 2003) and London, Ontario (Weinshenker et al., 1989a, b, 1991; Cottrell et al., 1999; Kremenchutzky et al., 1999), are based upon long-term longitudinal assessments at more or less close intervals. In their results' analysis, researchers formerly used to consider crude observed data only, gathered from patients who had reached the outcome criteria at the time of the survey. This strategy systematically led to underestimating the time interval to the outcome, as the censored patients (those lost to follow up or who had not yet reached the endpoint at the time of the survey) were not taken into account in the calculations. Modern survival techniques, which take account of data of censored patients, are therefore always to be preferred, as they provide more accurate estimates. Such techniques have been used in the long-term longitudinal studies of Lyon, Gothenburg and London (Confavreux, 1977; Confavreux et al., 1980, 2000, 2003; Broman et al., 1981; Weinshenker et al., 1989a, b, 1991a; Runmarker and Andersen, 1993; Cottrell et al., 1999; Kremenchutzky et al., 1999; Eriksson et al., 2003), and in several long-term studies with an essentially cross-sectional assessment (Riise et al., 1992; Kantarci et al., 1998; Amato and Ponziani, 2000; Myhr et al., 2001). Overall, the results from these different representative cohorts are consistent. They allow to make several statements with reasonable confidence for a representative population of patients. It takes a median time of 8, 20 and 30 years to reach the irreversible disability levels of DSS 4, 6 and 7, respectively. It takes much longer for cases with an exacerbating-remitting onset than in those with a progressive onset to reach levels of irreversible disability. An older age at onset of multiple sclerosis is associated with a more rapid accumulation of irreversible disability. Last, as lately shown in an Italian study, an older current age is associated with a shorter time to reach disability landmarks and a shift in the distribution of patients towards higher disability levels (Trojano et al., 2002).
These studies all focused on the assessment of the time to reach disability milestones in multiple sclerosis, regardless of the age at which patients reach these landmarks. This is somewhat surprising as onset of the relapsing-remitting and of the progressive phases of multiple sclerosis has repeatedly been demonstrated to be age-related, independently of the overall course of the disease, be it relapsing-remitting, secondary-progressive or progressive from onset (Fog and Linneman, 1970; Leibowitz and Alter, 1973; Confavreux, 1977; Poser, 1978; Confavreux et al., 1980; Minderhoud et al., 1988; Cottrel et al., 1999; Kremenchutzky et al., 1999). On average, mean age at the onset of the relapsing-remitting phase is 30 years whilst it is 38 years for the onset of the progressive phase. Furthermore, we have lately shown that clinically detectable relapses, whenever they may occur, have only a marginal effect on the accumulation of disability and that once a clinically detectable threshold of irreversible disability has been reached, the disease enters a final common pathway where subsequent accumulation of disability progresses at a similar rate independently of the initial course of multiple sclerosis, be it exacerbating-remitting or progressive (Confavreux et al., 2000, 2003). Therefore, cases with an exacerbating-remitting initial course and cases with a progressive onset differ with respect to the time from the onset of multiple sclerosis to reach disability milestones, whereas they are similar regarding the time course accumulation of disability further to the reaching of the clinically detectable threshold of irreversible disability. This prompted us to test the hypothesis whether these differences and similarities could reflect differences in ages at onset of multiple sclerosis and similarities in ages at reaching disability milestones in these two settings. For this purpose, we have referred to the Lyon Multiple Sclerosis Cohort, which is a unique natural history database both in terms of its size and the quantity of data gathered since five decades.
Methods
Patient population and data collection
Patients were identified through the Lyon Multiple Sclerosis Cohort which was established in the Lyon Clinique de Neurologie in 1957. Our clinic serves as the single reference centre for diagnosis confirmation, follow-up and treatment of multiple sclerosis patients for Lyon City and the Rhône-Alpes Region (Confavreux et al., 2000, 2003). Lyon is located within the ‘département du Rhône’ which had 1 575 000 inhabitants in 1999. The Rhône-Alpes Region is made of eight ‘départements’ (Ain, Ardèche, Drôme, Isère, Loire, Rhône, Savoie, Haute-Savoie) and counted 5 634 000 inhabitants in 1999. Its population is mainly Caucasian with some admixture, in the last decades, from the former French colonies in North Africa (Algeria, Morocco, Tunisia). This being said, the overall population in the area can be considered as essentially stable. The cohort includes all the patients with a diagnosis of multiple sclerosis examined at least once at the clinic. Data were computerized in 1976 and have been entered on the European Database for Multiple Sclerosis (EDMUS) software since 1990 (Confavreux et al., 1992).
Individual case reports include identification and demographic data, medical history, key-episodes in the multiple sclerosis course (relapses, onset of the progressive phase, dates of assignment of the successive scores of irreversible disability), biological, electrophysiological and neuro-imaging data and treatment. Data are entered retrospectively when the patient is first seen at the clinic. Thereafter, they are collected prospectively whenever the patient returns, usually on a yearly basis. New data are automatically checked by the system for their consistency with older information. Confidentiality and safety of the data are ensured in keeping with the recommendations of the French Commission Nationale Informatique et Libertés which also provides approval. All patients give informed consent for having their data saved in the database.
Definition of cases and assessment of patients
By April 1997, a cohort of 2021 patients had been included in the database. At that time, the database was locked for the purpose of epidemiological studies. Diagnosis of multiple sclerosis was established according to Poser's classification (Poser et al., 1983).
A relapse of multiple sclerosis was defined as the occurrence, the recurrence, or the worsening of symptoms of neurological dysfunction lasting over 24 h and usually ending up in partial or complete remission (Confavreux et al., 1992; Lublin and Reingold, 1996). Fatigue alone and transient fever-related worsening of symptoms were not considered as a relapse. Symptoms occurring within a month were considered as part of the same relapse. The progressive phase was defined as the steady worsening of symptoms and signs for at least 6 months, whether superimposed with relapses or not (Schumacher et al., 1965). Once started, the progressive phase continues throughout the disease though occasional plateaus and temporary minor improvements may be observed (Lublin and Reingold, 1996). Course of the disease was categorized according to acknowledged classifications (Confavreux et al., 1992; Lublin and Reingold, 1996). Initial course was considered as exacerbating-remitting or progressive. Overall course was classified relapsing-remitting when the disease exhibited only relapses and remissions; secondary progressive when an initial relapsing-remitting phase was followed by a progressive phase whether superimposed with relapses or not; progressive from onset when the progressive phase, whether superimposed with relapses or not, took place right from disease onset. Dates of onset of multiple sclerosis, of relapses, and of onset of the progressive phase were systematically assessed for each patient whenever appropriate.
The Kurtzke Disability Status Scale score was recorded at each visit to determine the extent of disability (Kurtzke, 1961, 1983). We focused on scores which could be easily identified, even when interviewing the patient retrospectively. A score of 4 corresponds to limited walking ability but without aid or rest for >500 m; a score of 6 corresponds to ability to walk with unilateral support no greater than 100 m without rest; and a score of 7 corresponds to the ability to walk no greater than 10 m without rest while leaning against a wall or holding onto furniture for support. Disability was defined as irreversible when a given score persisted at least 6 months, excluding transient worsening of disability related to relapses. By definition, when a given score of irreversible disability had been assigned to a given patient, all the scores of disability that could be subsequently assessed during the follow-up of the patient were either equal to or higher than that score. For each patient, the date of assignment to a given score of irreversible disability was assessed whenever appropriate.
Statistical analysis
End-points were ages at the time of assignment of an irreversible score of DSS 4, DSS 6 and DSS 7. They were first estimated for the entire patient cohort. In a second approach, they were estimated following stratification according to the initial course of the disease, and to other clinical variables. Age can be considered as a survival data. This is the time interval from birth to assignment of the chosen disability scores. Whenever the end-points had not been reached, data were censored at the age at the last visit. All the estimates were made using the Kaplan–Meier technique and displayed as cumulative probabilities to reach the end-point under study at a given age. Survival curves were compared using the log-rank test. Cox models were used for continuous data and for multivariate analyses. All computations were performed using SPSS for Windows, version 11.0.
Results
Clinical and demographic characteristics
Among the 2021 patients potentially eligible, 170 classified as possible multiple sclerosis only (Poser et al., 1983) and seven whose initial symptoms were unknown were excluded. The baseline characteristics of the remaining 1844 patients with a diagnosis of definite or probable multiple sclerosis have already been described (Confavreux et al., 2000, 2003). During the follow-up of the 1844 patients, a total of 1026 patients (56%), 595 (32%) and 380 (21%) reached the end-point of DSS 4, DSS 6 and DSS 7, respectively.
Age at time of assignment of irreversible disability levels
For the whole population of 1844 patients, the Kaplan–Meier analysis provided estimates of the median age at time of assignment of an irreversible score of disability. This age was 44.3 years (95% CI, 43.3–45.2) for DSS 4, 54.7 years (95% CI, 53.5–55.8) for DSS 6 and 63.1 years (95% CI, 61.0–65.1) for DSS 7 (Table 1).
Kaplan–Meier estimates of the age of the patients at the time of assignment of the irreversible disability scores of DSS 4, DSS 6, and DSS 7 among 1844 patients with multiple sclerosis
| Variable . | No. of patients (N = 1844) . | Age at assignment of DSS 4* . | . | . | . | Age at assignment of DSS 6 * . | . | . | . | Age at assignment of DSS 7 * . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | |||||||||||||
| Overall | 1844 | 44.3 | 34.8/52.6 | 44 | NA | 54.7 | 43.8/65.5 | 68 | NA | 63.1 | 51.2/74.8 | 79 | NA | |||||||||||||
| [43.3–45.2] | [53.5–55.8] | [61.0–65.1] | ||||||||||||||||||||||||
| Initial course of multiple sclerosis | ||||||||||||||||||||||||||
| Exacerbating remitting | 1562 | 44.8 | 35.2/54.9 | 52 | Reference | 55.3 | 44.7/70.3 | 73 | Reference | 62.8 | 51.5/NA | 82 | Reference | |||||||||||||
| [43.8–45.9] | [54.2–56.7] | [60.3–65.4] | ||||||||||||||||||||||||
| Progressive | 282 | 42.1 | 32.9/49.4 | 4 | <0.001 | 53.0 | 41.5/63.2 | 40 | 0.002 | 63.1 | 49.2/70.5 | 64 | 0.24 | |||||||||||||
| [40.2–44.0] | [51.1–54.9] | [60.0–66.2] | ||||||||||||||||||||||||
| Gender | ||||||||||||||||||||||||||
| Male | 657 | 42.2 | 34.3/51.8 | 40 | Reference | 52.1 | 41.6/63.6 | 63 | Reference | 61.8 | 47.8/74.8 | 77 | Reference | |||||||||||||
| [41.0–43.4] | [49.3–54.9] | [58.1–65.6] | ||||||||||||||||||||||||
| Female | 1187 | 46.0 | 35.4/53.4 | 47 | 0.003 | 56.1 | 46.1/65.8 | 70 | <0.001 | 63.1 | 53.7/82.1 | 81 | 0.012 | |||||||||||||
| [44.9–47.1] | [54.5–57.6] | [62.1–65.4] | ||||||||||||||||||||||||
| Age at onset of multiple sclerosis | ||||||||||||||||||||||||||
| 0–19 years | 216 | 31.6 | 22.3/44.7 | 50 | Reference | 46.8 | 31.8/58.2 | 70 | Reference | 50.3 | 34.1/NA | 78 | Reference | |||||||||||||
| [28.3–34.9] | [41.6–52.1] | [42.6–57.6] | ||||||||||||||||||||||||
| 20–29 years | 690 | 36.4 | 29.4/48.6 | 51 | <0.001 | 51.2 | 37.0/69.8 | 73 | <0.001 | 58.2 | 42.3/71.2 | 82 | <0.001 | |||||||||||||
| [34.9–38.0] | [47.6–54.8] | [50.1–66.3] | ||||||||||||||||||||||||
| 30–39 years | 558 | 42.8 | 37.3/51.8 | 44 | <0.001 | 52.5 | 43.1/74.8 | 67 | <0.001 | 59.1 | 49.3/NA | 79 | <0.001 | |||||||||||||
| [41.6–44.0] | [50.3–54.7] | [54.4–63.8] | ||||||||||||||||||||||||
| 40–49 years | 272 | 48.2 | 44.8/51.8 | 32 | <0.001 | 56.7 | 52.0/65.6 | 61 | <0.001 | 64.8 | 56.8/NA | 79 | <0.001 | |||||||||||||
| [47.3–49.0] | [54.3–59.0] | [61.4–68.3] | ||||||||||||||||||||||||
| More than 50 years | 108 | 57.1 | 53.2/60.7 | 24 | <0.001 | 63.8 | 58.8/71.6 | 50 | <0.001 | 70.1 | 62.8/74.8 | 70 | <0.001 | |||||||||||||
| [55.6–58.5] | [62.7–65.0] | [64.7–75.4] | ||||||||||||||||||||||||
| <0.001 | <0.001 | < 0.001 | ||||||||||||||||||||||||
| Initial symptoms | ||||||||||||||||||||||||||
| Overall | ||||||||||||||||||||||||||
| Isolated optic neuritis | 335 | 46.1 | 37.3/55.6 | 53 | Reference | 57.8 | 45.7/65.9 | 73 | Reference | 65.8 | 52.4/NA | 84 | Reference | |||||||||||||
| [43.6–48.6] | [53.7–61.8] | [? - ?] | ||||||||||||||||||||||||
| Isolated brainstem | 159 | 42.7 | 35.0/52.2 | 52 | 0.36 | 54.9 | 44.5/71.6 | 72 | 0.78 | 61.1 | 47.2/NA | 79 | 0.13 | |||||||||||||
| dysfunction | [39.5–45.8] | [52.3–57.5] | [50.2–71.9] | |||||||||||||||||||||||
| Isolated dysfunction | 964 | 43.8 | 34.8/51.3 | 49 | 0.01 | 54.0 | 43.3/64.3 | 72 | 0.11 | 62.8 | 51.5/82.1 | 82 | 0.39 | |||||||||||||
| of long tracts | [42.6–45.0] | [52.5–55.5] | [57.7–64.3] | |||||||||||||||||||||||
| Combination of | 386 | 44.3 | 33.3/54.7 | 38 | 0.11 | 54.8 | 43.8/64.5 | 63 | 0.25 | 61.0 | 51.0/72.5 | 77 | 0.27 | |||||||||||||
| symptoms | [42.2–46.3] | [51.3–58.4] | [60.6–65.1] | |||||||||||||||||||||||
| 0.10 | 0.36 | 0.57 | ||||||||||||||||||||||||
| Long tracts involvement | ||||||||||||||||||||||||||
| Yes | 1287 | 43.9 | 34.6/52.1 | 41 | 0.03 | 54.2 | 43.3/64.3 | 65 | 0.06 | 62.3 | 51.4/72.5 | 78 | 0.79 | |||||||||||||
| [42.8–45.0] | [52.8–55.5] | [60.2–64.3] | ||||||||||||||||||||||||
| No | 557 | 44.8 | 36.0/55.6 | 52 | Reference | 56.7 | 45.0/71.6 | 74 | Reference | 65.8 | 51.0/NA | 82 | ||||||||||||||
| [43.1–46.6] | Reference | [52.2–61.1] | [59.6–71.9] | |||||||||||||||||||||||
| Brainstem involvement | ||||||||||||||||||||||||||
| Yes | 411 | 44.7 | 33.3/54.2 | 50 | 0.99 | 55.1 | 43.3/71.6 | 71 | 0.75 | 61.1 | 49.3/74.8 | 80 | 0.45 | |||||||||||||
| [42.7–46.7] | [51.6–58.6] | [55.0–67.1] | ||||||||||||||||||||||||
| No | 1433 | 44.1 | 35.2/52.3 | 43 | Reference | 54.5 | 44.0/64.8 | 67 | Reference | 63.3 | 51.5/82.1 | 79 | Reference | |||||||||||||
| [43.0–45.1] | [53.1–55.9] | [61.4–65.1] | ||||||||||||||||||||||||
| Optic neuritis | ||||||||||||||||||||||||||
| Yes | 476 | 45.5 | 36.5/55.6 | 53 | 0.02 | 56.7 | 45.6/NA | 75 | 0.11 | 65.8 | 51.0/NA | 84 | 0.26 | |||||||||||||
| [43.7–47.3] | [53.0–60.3] | [? - ?] | ||||||||||||||||||||||||
| No | 1368 | 43.9 | 34.5/52.1 | 41 | Reference | 54.2 | 43.0/64.5 | 65 | Reference | 62.3 | 51.2/74.8 | 78 | Reference | |||||||||||||
| [42.9–45.0] | [52.8–55.5] | [60.3–64.2] | ||||||||||||||||||||||||
| Variable . | No. of patients (N = 1844) . | Age at assignment of DSS 4* . | . | . | . | Age at assignment of DSS 6 * . | . | . | . | Age at assignment of DSS 7 * . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | |||||||||||||
| Overall | 1844 | 44.3 | 34.8/52.6 | 44 | NA | 54.7 | 43.8/65.5 | 68 | NA | 63.1 | 51.2/74.8 | 79 | NA | |||||||||||||
| [43.3–45.2] | [53.5–55.8] | [61.0–65.1] | ||||||||||||||||||||||||
| Initial course of multiple sclerosis | ||||||||||||||||||||||||||
| Exacerbating remitting | 1562 | 44.8 | 35.2/54.9 | 52 | Reference | 55.3 | 44.7/70.3 | 73 | Reference | 62.8 | 51.5/NA | 82 | Reference | |||||||||||||
| [43.8–45.9] | [54.2–56.7] | [60.3–65.4] | ||||||||||||||||||||||||
| Progressive | 282 | 42.1 | 32.9/49.4 | 4 | <0.001 | 53.0 | 41.5/63.2 | 40 | 0.002 | 63.1 | 49.2/70.5 | 64 | 0.24 | |||||||||||||
| [40.2–44.0] | [51.1–54.9] | [60.0–66.2] | ||||||||||||||||||||||||
| Gender | ||||||||||||||||||||||||||
| Male | 657 | 42.2 | 34.3/51.8 | 40 | Reference | 52.1 | 41.6/63.6 | 63 | Reference | 61.8 | 47.8/74.8 | 77 | Reference | |||||||||||||
| [41.0–43.4] | [49.3–54.9] | [58.1–65.6] | ||||||||||||||||||||||||
| Female | 1187 | 46.0 | 35.4/53.4 | 47 | 0.003 | 56.1 | 46.1/65.8 | 70 | <0.001 | 63.1 | 53.7/82.1 | 81 | 0.012 | |||||||||||||
| [44.9–47.1] | [54.5–57.6] | [62.1–65.4] | ||||||||||||||||||||||||
| Age at onset of multiple sclerosis | ||||||||||||||||||||||||||
| 0–19 years | 216 | 31.6 | 22.3/44.7 | 50 | Reference | 46.8 | 31.8/58.2 | 70 | Reference | 50.3 | 34.1/NA | 78 | Reference | |||||||||||||
| [28.3–34.9] | [41.6–52.1] | [42.6–57.6] | ||||||||||||||||||||||||
| 20–29 years | 690 | 36.4 | 29.4/48.6 | 51 | <0.001 | 51.2 | 37.0/69.8 | 73 | <0.001 | 58.2 | 42.3/71.2 | 82 | <0.001 | |||||||||||||
| [34.9–38.0] | [47.6–54.8] | [50.1–66.3] | ||||||||||||||||||||||||
| 30–39 years | 558 | 42.8 | 37.3/51.8 | 44 | <0.001 | 52.5 | 43.1/74.8 | 67 | <0.001 | 59.1 | 49.3/NA | 79 | <0.001 | |||||||||||||
| [41.6–44.0] | [50.3–54.7] | [54.4–63.8] | ||||||||||||||||||||||||
| 40–49 years | 272 | 48.2 | 44.8/51.8 | 32 | <0.001 | 56.7 | 52.0/65.6 | 61 | <0.001 | 64.8 | 56.8/NA | 79 | <0.001 | |||||||||||||
| [47.3–49.0] | [54.3–59.0] | [61.4–68.3] | ||||||||||||||||||||||||
| More than 50 years | 108 | 57.1 | 53.2/60.7 | 24 | <0.001 | 63.8 | 58.8/71.6 | 50 | <0.001 | 70.1 | 62.8/74.8 | 70 | <0.001 | |||||||||||||
| [55.6–58.5] | [62.7–65.0] | [64.7–75.4] | ||||||||||||||||||||||||
| <0.001 | <0.001 | < 0.001 | ||||||||||||||||||||||||
| Initial symptoms | ||||||||||||||||||||||||||
| Overall | ||||||||||||||||||||||||||
| Isolated optic neuritis | 335 | 46.1 | 37.3/55.6 | 53 | Reference | 57.8 | 45.7/65.9 | 73 | Reference | 65.8 | 52.4/NA | 84 | Reference | |||||||||||||
| [43.6–48.6] | [53.7–61.8] | [? - ?] | ||||||||||||||||||||||||
| Isolated brainstem | 159 | 42.7 | 35.0/52.2 | 52 | 0.36 | 54.9 | 44.5/71.6 | 72 | 0.78 | 61.1 | 47.2/NA | 79 | 0.13 | |||||||||||||
| dysfunction | [39.5–45.8] | [52.3–57.5] | [50.2–71.9] | |||||||||||||||||||||||
| Isolated dysfunction | 964 | 43.8 | 34.8/51.3 | 49 | 0.01 | 54.0 | 43.3/64.3 | 72 | 0.11 | 62.8 | 51.5/82.1 | 82 | 0.39 | |||||||||||||
| of long tracts | [42.6–45.0] | [52.5–55.5] | [57.7–64.3] | |||||||||||||||||||||||
| Combination of | 386 | 44.3 | 33.3/54.7 | 38 | 0.11 | 54.8 | 43.8/64.5 | 63 | 0.25 | 61.0 | 51.0/72.5 | 77 | 0.27 | |||||||||||||
| symptoms | [42.2–46.3] | [51.3–58.4] | [60.6–65.1] | |||||||||||||||||||||||
| 0.10 | 0.36 | 0.57 | ||||||||||||||||||||||||
| Long tracts involvement | ||||||||||||||||||||||||||
| Yes | 1287 | 43.9 | 34.6/52.1 | 41 | 0.03 | 54.2 | 43.3/64.3 | 65 | 0.06 | 62.3 | 51.4/72.5 | 78 | 0.79 | |||||||||||||
| [42.8–45.0] | [52.8–55.5] | [60.2–64.3] | ||||||||||||||||||||||||
| No | 557 | 44.8 | 36.0/55.6 | 52 | Reference | 56.7 | 45.0/71.6 | 74 | Reference | 65.8 | 51.0/NA | 82 | ||||||||||||||
| [43.1–46.6] | Reference | [52.2–61.1] | [59.6–71.9] | |||||||||||||||||||||||
| Brainstem involvement | ||||||||||||||||||||||||||
| Yes | 411 | 44.7 | 33.3/54.2 | 50 | 0.99 | 55.1 | 43.3/71.6 | 71 | 0.75 | 61.1 | 49.3/74.8 | 80 | 0.45 | |||||||||||||
| [42.7–46.7] | [51.6–58.6] | [55.0–67.1] | ||||||||||||||||||||||||
| No | 1433 | 44.1 | 35.2/52.3 | 43 | Reference | 54.5 | 44.0/64.8 | 67 | Reference | 63.3 | 51.5/82.1 | 79 | Reference | |||||||||||||
| [43.0–45.1] | [53.1–55.9] | [61.4–65.1] | ||||||||||||||||||||||||
| Optic neuritis | ||||||||||||||||||||||||||
| Yes | 476 | 45.5 | 36.5/55.6 | 53 | 0.02 | 56.7 | 45.6/NA | 75 | 0.11 | 65.8 | 51.0/NA | 84 | 0.26 | |||||||||||||
| [43.7–47.3] | [53.0–60.3] | [? - ?] | ||||||||||||||||||||||||
| No | 1368 | 43.9 | 34.5/52.1 | 41 | Reference | 54.2 | 43.0/64.5 | 65 | Reference | 62.3 | 51.2/74.8 | 78 | Reference | |||||||||||||
| [42.9–45.0] | [52.8–55.5] | [60.3–64.2] | ||||||||||||||||||||||||
The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke, 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. A given score of disability was defined as irreversible when a patient had had that score or more for at least 6 months, excluding any transient worsening of disability related to relapses.
P-values were calculated with use of the log-rank test.
Data on the patients who did not reach an end point were censored at the time of the last clinic visit.
Kaplan–Meier estimates of the age of the patients at the time of assignment of the irreversible disability scores of DSS 4, DSS 6, and DSS 7 among 1844 patients with multiple sclerosis
| Variable . | No. of patients (N = 1844) . | Age at assignment of DSS 4* . | . | . | . | Age at assignment of DSS 6 * . | . | . | . | Age at assignment of DSS 7 * . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | |||||||||||||
| Overall | 1844 | 44.3 | 34.8/52.6 | 44 | NA | 54.7 | 43.8/65.5 | 68 | NA | 63.1 | 51.2/74.8 | 79 | NA | |||||||||||||
| [43.3–45.2] | [53.5–55.8] | [61.0–65.1] | ||||||||||||||||||||||||
| Initial course of multiple sclerosis | ||||||||||||||||||||||||||
| Exacerbating remitting | 1562 | 44.8 | 35.2/54.9 | 52 | Reference | 55.3 | 44.7/70.3 | 73 | Reference | 62.8 | 51.5/NA | 82 | Reference | |||||||||||||
| [43.8–45.9] | [54.2–56.7] | [60.3–65.4] | ||||||||||||||||||||||||
| Progressive | 282 | 42.1 | 32.9/49.4 | 4 | <0.001 | 53.0 | 41.5/63.2 | 40 | 0.002 | 63.1 | 49.2/70.5 | 64 | 0.24 | |||||||||||||
| [40.2–44.0] | [51.1–54.9] | [60.0–66.2] | ||||||||||||||||||||||||
| Gender | ||||||||||||||||||||||||||
| Male | 657 | 42.2 | 34.3/51.8 | 40 | Reference | 52.1 | 41.6/63.6 | 63 | Reference | 61.8 | 47.8/74.8 | 77 | Reference | |||||||||||||
| [41.0–43.4] | [49.3–54.9] | [58.1–65.6] | ||||||||||||||||||||||||
| Female | 1187 | 46.0 | 35.4/53.4 | 47 | 0.003 | 56.1 | 46.1/65.8 | 70 | <0.001 | 63.1 | 53.7/82.1 | 81 | 0.012 | |||||||||||||
| [44.9–47.1] | [54.5–57.6] | [62.1–65.4] | ||||||||||||||||||||||||
| Age at onset of multiple sclerosis | ||||||||||||||||||||||||||
| 0–19 years | 216 | 31.6 | 22.3/44.7 | 50 | Reference | 46.8 | 31.8/58.2 | 70 | Reference | 50.3 | 34.1/NA | 78 | Reference | |||||||||||||
| [28.3–34.9] | [41.6–52.1] | [42.6–57.6] | ||||||||||||||||||||||||
| 20–29 years | 690 | 36.4 | 29.4/48.6 | 51 | <0.001 | 51.2 | 37.0/69.8 | 73 | <0.001 | 58.2 | 42.3/71.2 | 82 | <0.001 | |||||||||||||
| [34.9–38.0] | [47.6–54.8] | [50.1–66.3] | ||||||||||||||||||||||||
| 30–39 years | 558 | 42.8 | 37.3/51.8 | 44 | <0.001 | 52.5 | 43.1/74.8 | 67 | <0.001 | 59.1 | 49.3/NA | 79 | <0.001 | |||||||||||||
| [41.6–44.0] | [50.3–54.7] | [54.4–63.8] | ||||||||||||||||||||||||
| 40–49 years | 272 | 48.2 | 44.8/51.8 | 32 | <0.001 | 56.7 | 52.0/65.6 | 61 | <0.001 | 64.8 | 56.8/NA | 79 | <0.001 | |||||||||||||
| [47.3–49.0] | [54.3–59.0] | [61.4–68.3] | ||||||||||||||||||||||||
| More than 50 years | 108 | 57.1 | 53.2/60.7 | 24 | <0.001 | 63.8 | 58.8/71.6 | 50 | <0.001 | 70.1 | 62.8/74.8 | 70 | <0.001 | |||||||||||||
| [55.6–58.5] | [62.7–65.0] | [64.7–75.4] | ||||||||||||||||||||||||
| <0.001 | <0.001 | < 0.001 | ||||||||||||||||||||||||
| Initial symptoms | ||||||||||||||||||||||||||
| Overall | ||||||||||||||||||||||||||
| Isolated optic neuritis | 335 | 46.1 | 37.3/55.6 | 53 | Reference | 57.8 | 45.7/65.9 | 73 | Reference | 65.8 | 52.4/NA | 84 | Reference | |||||||||||||
| [43.6–48.6] | [53.7–61.8] | [? - ?] | ||||||||||||||||||||||||
| Isolated brainstem | 159 | 42.7 | 35.0/52.2 | 52 | 0.36 | 54.9 | 44.5/71.6 | 72 | 0.78 | 61.1 | 47.2/NA | 79 | 0.13 | |||||||||||||
| dysfunction | [39.5–45.8] | [52.3–57.5] | [50.2–71.9] | |||||||||||||||||||||||
| Isolated dysfunction | 964 | 43.8 | 34.8/51.3 | 49 | 0.01 | 54.0 | 43.3/64.3 | 72 | 0.11 | 62.8 | 51.5/82.1 | 82 | 0.39 | |||||||||||||
| of long tracts | [42.6–45.0] | [52.5–55.5] | [57.7–64.3] | |||||||||||||||||||||||
| Combination of | 386 | 44.3 | 33.3/54.7 | 38 | 0.11 | 54.8 | 43.8/64.5 | 63 | 0.25 | 61.0 | 51.0/72.5 | 77 | 0.27 | |||||||||||||
| symptoms | [42.2–46.3] | [51.3–58.4] | [60.6–65.1] | |||||||||||||||||||||||
| 0.10 | 0.36 | 0.57 | ||||||||||||||||||||||||
| Long tracts involvement | ||||||||||||||||||||||||||
| Yes | 1287 | 43.9 | 34.6/52.1 | 41 | 0.03 | 54.2 | 43.3/64.3 | 65 | 0.06 | 62.3 | 51.4/72.5 | 78 | 0.79 | |||||||||||||
| [42.8–45.0] | [52.8–55.5] | [60.2–64.3] | ||||||||||||||||||||||||
| No | 557 | 44.8 | 36.0/55.6 | 52 | Reference | 56.7 | 45.0/71.6 | 74 | Reference | 65.8 | 51.0/NA | 82 | ||||||||||||||
| [43.1–46.6] | Reference | [52.2–61.1] | [59.6–71.9] | |||||||||||||||||||||||
| Brainstem involvement | ||||||||||||||||||||||||||
| Yes | 411 | 44.7 | 33.3/54.2 | 50 | 0.99 | 55.1 | 43.3/71.6 | 71 | 0.75 | 61.1 | 49.3/74.8 | 80 | 0.45 | |||||||||||||
| [42.7–46.7] | [51.6–58.6] | [55.0–67.1] | ||||||||||||||||||||||||
| No | 1433 | 44.1 | 35.2/52.3 | 43 | Reference | 54.5 | 44.0/64.8 | 67 | Reference | 63.3 | 51.5/82.1 | 79 | Reference | |||||||||||||
| [43.0–45.1] | [53.1–55.9] | [61.4–65.1] | ||||||||||||||||||||||||
| Optic neuritis | ||||||||||||||||||||||||||
| Yes | 476 | 45.5 | 36.5/55.6 | 53 | 0.02 | 56.7 | 45.6/NA | 75 | 0.11 | 65.8 | 51.0/NA | 84 | 0.26 | |||||||||||||
| [43.7–47.3] | [53.0–60.3] | [? - ?] | ||||||||||||||||||||||||
| No | 1368 | 43.9 | 34.5/52.1 | 41 | Reference | 54.2 | 43.0/64.5 | 65 | Reference | 62.3 | 51.2/74.8 | 78 | Reference | |||||||||||||
| [42.9–45.0] | [52.8–55.5] | [60.3–64.2] | ||||||||||||||||||||||||
| Variable . | No. of patients (N = 1844) . | Age at assignment of DSS 4* . | . | . | . | Age at assignment of DSS 6 * . | . | . | . | Age at assignment of DSS 7 * . | . | . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | Median - yr [95% CI] . | 25th/75th percentiles . | Patients who did not reach the end point (%) # . | P-value ** . | |||||||||||||
| Overall | 1844 | 44.3 | 34.8/52.6 | 44 | NA | 54.7 | 43.8/65.5 | 68 | NA | 63.1 | 51.2/74.8 | 79 | NA | |||||||||||||
| [43.3–45.2] | [53.5–55.8] | [61.0–65.1] | ||||||||||||||||||||||||
| Initial course of multiple sclerosis | ||||||||||||||||||||||||||
| Exacerbating remitting | 1562 | 44.8 | 35.2/54.9 | 52 | Reference | 55.3 | 44.7/70.3 | 73 | Reference | 62.8 | 51.5/NA | 82 | Reference | |||||||||||||
| [43.8–45.9] | [54.2–56.7] | [60.3–65.4] | ||||||||||||||||||||||||
| Progressive | 282 | 42.1 | 32.9/49.4 | 4 | <0.001 | 53.0 | 41.5/63.2 | 40 | 0.002 | 63.1 | 49.2/70.5 | 64 | 0.24 | |||||||||||||
| [40.2–44.0] | [51.1–54.9] | [60.0–66.2] | ||||||||||||||||||||||||
| Gender | ||||||||||||||||||||||||||
| Male | 657 | 42.2 | 34.3/51.8 | 40 | Reference | 52.1 | 41.6/63.6 | 63 | Reference | 61.8 | 47.8/74.8 | 77 | Reference | |||||||||||||
| [41.0–43.4] | [49.3–54.9] | [58.1–65.6] | ||||||||||||||||||||||||
| Female | 1187 | 46.0 | 35.4/53.4 | 47 | 0.003 | 56.1 | 46.1/65.8 | 70 | <0.001 | 63.1 | 53.7/82.1 | 81 | 0.012 | |||||||||||||
| [44.9–47.1] | [54.5–57.6] | [62.1–65.4] | ||||||||||||||||||||||||
| Age at onset of multiple sclerosis | ||||||||||||||||||||||||||
| 0–19 years | 216 | 31.6 | 22.3/44.7 | 50 | Reference | 46.8 | 31.8/58.2 | 70 | Reference | 50.3 | 34.1/NA | 78 | Reference | |||||||||||||
| [28.3–34.9] | [41.6–52.1] | [42.6–57.6] | ||||||||||||||||||||||||
| 20–29 years | 690 | 36.4 | 29.4/48.6 | 51 | <0.001 | 51.2 | 37.0/69.8 | 73 | <0.001 | 58.2 | 42.3/71.2 | 82 | <0.001 | |||||||||||||
| [34.9–38.0] | [47.6–54.8] | [50.1–66.3] | ||||||||||||||||||||||||
| 30–39 years | 558 | 42.8 | 37.3/51.8 | 44 | <0.001 | 52.5 | 43.1/74.8 | 67 | <0.001 | 59.1 | 49.3/NA | 79 | <0.001 | |||||||||||||
| [41.6–44.0] | [50.3–54.7] | [54.4–63.8] | ||||||||||||||||||||||||
| 40–49 years | 272 | 48.2 | 44.8/51.8 | 32 | <0.001 | 56.7 | 52.0/65.6 | 61 | <0.001 | 64.8 | 56.8/NA | 79 | <0.001 | |||||||||||||
| [47.3–49.0] | [54.3–59.0] | [61.4–68.3] | ||||||||||||||||||||||||
| More than 50 years | 108 | 57.1 | 53.2/60.7 | 24 | <0.001 | 63.8 | 58.8/71.6 | 50 | <0.001 | 70.1 | 62.8/74.8 | 70 | <0.001 | |||||||||||||
| [55.6–58.5] | [62.7–65.0] | [64.7–75.4] | ||||||||||||||||||||||||
| <0.001 | <0.001 | < 0.001 | ||||||||||||||||||||||||
| Initial symptoms | ||||||||||||||||||||||||||
| Overall | ||||||||||||||||||||||||||
| Isolated optic neuritis | 335 | 46.1 | 37.3/55.6 | 53 | Reference | 57.8 | 45.7/65.9 | 73 | Reference | 65.8 | 52.4/NA | 84 | Reference | |||||||||||||
| [43.6–48.6] | [53.7–61.8] | [? - ?] | ||||||||||||||||||||||||
| Isolated brainstem | 159 | 42.7 | 35.0/52.2 | 52 | 0.36 | 54.9 | 44.5/71.6 | 72 | 0.78 | 61.1 | 47.2/NA | 79 | 0.13 | |||||||||||||
| dysfunction | [39.5–45.8] | [52.3–57.5] | [50.2–71.9] | |||||||||||||||||||||||
| Isolated dysfunction | 964 | 43.8 | 34.8/51.3 | 49 | 0.01 | 54.0 | 43.3/64.3 | 72 | 0.11 | 62.8 | 51.5/82.1 | 82 | 0.39 | |||||||||||||
| of long tracts | [42.6–45.0] | [52.5–55.5] | [57.7–64.3] | |||||||||||||||||||||||
| Combination of | 386 | 44.3 | 33.3/54.7 | 38 | 0.11 | 54.8 | 43.8/64.5 | 63 | 0.25 | 61.0 | 51.0/72.5 | 77 | 0.27 | |||||||||||||
| symptoms | [42.2–46.3] | [51.3–58.4] | [60.6–65.1] | |||||||||||||||||||||||
| 0.10 | 0.36 | 0.57 | ||||||||||||||||||||||||
| Long tracts involvement | ||||||||||||||||||||||||||
| Yes | 1287 | 43.9 | 34.6/52.1 | 41 | 0.03 | 54.2 | 43.3/64.3 | 65 | 0.06 | 62.3 | 51.4/72.5 | 78 | 0.79 | |||||||||||||
| [42.8–45.0] | [52.8–55.5] | [60.2–64.3] | ||||||||||||||||||||||||
| No | 557 | 44.8 | 36.0/55.6 | 52 | Reference | 56.7 | 45.0/71.6 | 74 | Reference | 65.8 | 51.0/NA | 82 | ||||||||||||||
| [43.1–46.6] | Reference | [52.2–61.1] | [59.6–71.9] | |||||||||||||||||||||||
| Brainstem involvement | ||||||||||||||||||||||||||
| Yes | 411 | 44.7 | 33.3/54.2 | 50 | 0.99 | 55.1 | 43.3/71.6 | 71 | 0.75 | 61.1 | 49.3/74.8 | 80 | 0.45 | |||||||||||||
| [42.7–46.7] | [51.6–58.6] | [55.0–67.1] | ||||||||||||||||||||||||
| No | 1433 | 44.1 | 35.2/52.3 | 43 | Reference | 54.5 | 44.0/64.8 | 67 | Reference | 63.3 | 51.5/82.1 | 79 | Reference | |||||||||||||
| [43.0–45.1] | [53.1–55.9] | [61.4–65.1] | ||||||||||||||||||||||||
| Optic neuritis | ||||||||||||||||||||||||||
| Yes | 476 | 45.5 | 36.5/55.6 | 53 | 0.02 | 56.7 | 45.6/NA | 75 | 0.11 | 65.8 | 51.0/NA | 84 | 0.26 | |||||||||||||
| [43.7–47.3] | [53.0–60.3] | [? - ?] | ||||||||||||||||||||||||
| No | 1368 | 43.9 | 34.5/52.1 | 41 | Reference | 54.2 | 43.0/64.5 | 65 | Reference | 62.3 | 51.2/74.8 | 78 | Reference | |||||||||||||
| [42.9–45.0] | [52.8–55.5] | [60.3–64.2] | ||||||||||||||||||||||||
The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke, 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. A given score of disability was defined as irreversible when a patient had had that score or more for at least 6 months, excluding any transient worsening of disability related to relapses.
P-values were calculated with use of the log-rank test.
Data on the patients who did not reach an end point were censored at the time of the last clinic visit.
Influence of the initial course of multiple sclerosis
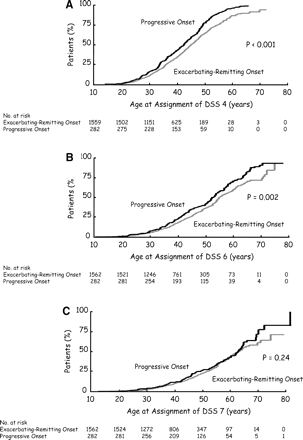
The 1562 patients with an exacerbating-remitting initial course were compared to the 282 patients with a progressive initial course of multiple sclerosis with respect to age at time of assignment of irreversible scores of disability. Patients with an exacerbating-remitting onset were older than those progressive from onset for assignment of DSS 4 (P < 0.001) and DSS 6 (P = 0.002). However, for both assignments, there was some overlap in the 95% CI of the median estimates. As for age at assignment of DSS 7, it was similar in both groups (P = 0.24) (Table 1 and Fig. 1).
Kaplan–Meier estimates of the age of the patients at the time of assignment of the irreversible scores of DSS 4 (A), DSS 6 (B) and DSS 7 (C) among 1844 patients with multiple sclerosis, according to the initial course of multiple sclerosis. (A) Among the 1562 patients with an exacerbating-remitting onset of multiple sclerosis, three did reach the score of 4 before the age of 10 years. The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. A given score of disability was defined as irreversible when a patient had had that score or more for at least 6 months, excluding any transient worsening of disability related to relapses.
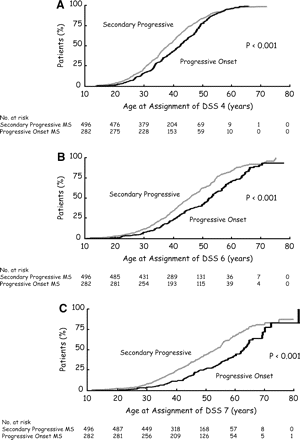
By contrast, when we analysed the subgroup of 496 patients with a secondary-progressive course among the whole group of 1562 patients with an exacerbating-remitting initial course, we found a significantly younger age at time of assignment of DSS 4 (median: 37.6 years; 95% CI, 36.1–39.1; P < 0.001), DSS 6 (median: 45.5 years; 95% CI, 43.6–47.4; P < 0.001) and DSS 7 (median: 53.3 years; 95% CI, 51.0–55.7; P < 0.001) in this subgroup by comparison to the group of 282 patients with a progressive initial course (Fig. 2).
Kaplan–Meier estimates of the age of the patients at the time of assignment of the irreversible scores of DSS 4 (A), DSS 6 (B) and DSS 7 (C) among 1844 patients with multiple sclerosis, according to a secondary progressive or a progressive from onset course of multiple sclerosis. The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. A given score of disability was defined as irreversible when a patient had had that score or more for at least 6 months, excluding any transient worsening of disability related to relapses.
Influence of other clinical variables
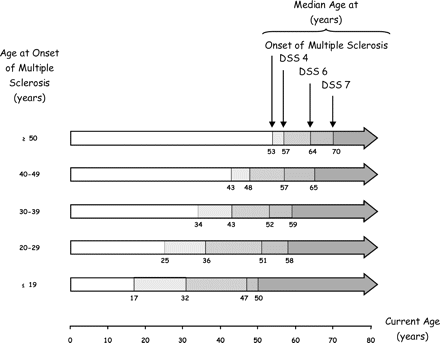
The analysis of the possible influence of other clinical factors essentially showed that male gender was associated with an earlier age at assignment of the irreversible disability landmarks. Similarly, the earlier the age at onset of multiple sclerosis, the earlier the age at assignment of the landmarks of disability (P < 0.001 for all the comparisons) (Fig. 3). By contrast, initial symptoms of multiple sclerosis essentially did not influence the age at the time of assignment of irreversible disability (Table 1). These results were confirmed by the multivariate analysis using Cox regression models, showing that a female gender, an older age at onset, and an exacerbating-remitting onset were independently associated with an older age at assignment of irreversible disability levels (Table 2).
Age at onset of multiple sclerosis and age at times of assignment of the irreversible disability scores of DSS 4, DSS 6 and DSS 7 among 1844 patients with multiple sclerosis. Each horizontal arrow represents a category of patients by age at onset of multiple sclerosis. The digits below the horizontal arrows indicate the median ages (years) for the corresponding category of patients at onset of multiple sclerosis (left), and at assignment of DSS 4 (middle left), of DSS 6 (middle right), and of DSS 7 (right). Ages are estimated by the Kaplan–Meier technique. The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. A given score of disability was defined as irreversible when a patient had had that score or more for at least 6 months, excluding any transient worsening of disability related to relapses.
Final Cox regression models of the age of the patients at the time of assignment of an irreversible disability score of DSS 4, DSS 6, and DSS 7 among 1844 patients with multiple sclerosis
| Variable | No. of patients (N = 1844) | Age at assignment of DSS 4* | Age at assignment of DSS 6* | Age at assignment of DSS 7* | ||||||||||
| Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | |||||||||
| Initial course of multiple sclerosis | ||||||||||||||
| Exacerbating-remitting | 1562 | – | Reference | – | Reference | – | Reference | |||||||
| Progressive | 282 | 3.89 | <0.001 | 2.72 | <0.001 | 2.39 | <0.001 | |||||||
| [3.25–4.65] | [2.21–3.35] | [1.84–3.10] | ||||||||||||
| Gender | ||||||||||||||
| Male | 657 | – | Reference | – | Reference | – | Reference | |||||||
| Female | 1187 | 0.85[0.75–0.96] | 0.01 | 0.75[0.63–0.88] | 0.001 | 0.74[0.60–0.91] | 0.001 | |||||||
| Age at onset of multiple sclerosis | ||||||||||||||
| 0–19 years | 216 | – | Reference | – | Reference | – | Reference | |||||||
| 20–29 years | 690 | 0.54[0.43–0.67] | <0.001 | 0.55[0.42–0.73] | <0.001 | 0.49[0.35–0.69] | <0.001 | |||||||
| 30–39 years | 558 | 0.23[0.18–0.28] | <0.001 | 0.29[0.22–0.39] | <0.001 | 0.24[0.17–0.34] | <0.001 | |||||||
| 40–49 years | 272 | 0.10[0.08–0.13] | <0.001 | 0.13[0.10–0.19] | <0.001 | 0.10[0.07–0.16] | <0.001 | |||||||
| More than 50 years | 108 | 0.04[0.03–0.05] | <0.001 | 0.07[0.05–0.11] | <0.001 | 0.06[0.04–0.10] | <0.001 | |||||||
| <0.001 | <0.001 | <0.001 | ||||||||||||
| Initial symptoms | ||||||||||||||
| Isolated dysfunction of long tracts | 964 | – | Reference | – | – | – | – | |||||||
| Isolated optic neuritis | 335 | 0.76[0.63–0.91] | 0.004 | – | – | – | – | |||||||
| Isolated brainstem dysfunction | 159 | 0.96[0.75–1.22] | 0.72 | – | – | – | – | |||||||
| Combination of symptoms | 386 | 1.03[0.88–1.22] | 0.71 | – | – | – | – | |||||||
| 0.02 | ||||||||||||||
| Variable | No. of patients (N = 1844) | Age at assignment of DSS 4* | Age at assignment of DSS 6* | Age at assignment of DSS 7* | ||||||||||
| Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | |||||||||
| Initial course of multiple sclerosis | ||||||||||||||
| Exacerbating-remitting | 1562 | – | Reference | – | Reference | – | Reference | |||||||
| Progressive | 282 | 3.89 | <0.001 | 2.72 | <0.001 | 2.39 | <0.001 | |||||||
| [3.25–4.65] | [2.21–3.35] | [1.84–3.10] | ||||||||||||
| Gender | ||||||||||||||
| Male | 657 | – | Reference | – | Reference | – | Reference | |||||||
| Female | 1187 | 0.85[0.75–0.96] | 0.01 | 0.75[0.63–0.88] | 0.001 | 0.74[0.60–0.91] | 0.001 | |||||||
| Age at onset of multiple sclerosis | ||||||||||||||
| 0–19 years | 216 | – | Reference | – | Reference | – | Reference | |||||||
| 20–29 years | 690 | 0.54[0.43–0.67] | <0.001 | 0.55[0.42–0.73] | <0.001 | 0.49[0.35–0.69] | <0.001 | |||||||
| 30–39 years | 558 | 0.23[0.18–0.28] | <0.001 | 0.29[0.22–0.39] | <0.001 | 0.24[0.17–0.34] | <0.001 | |||||||
| 40–49 years | 272 | 0.10[0.08–0.13] | <0.001 | 0.13[0.10–0.19] | <0.001 | 0.10[0.07–0.16] | <0.001 | |||||||
| More than 50 years | 108 | 0.04[0.03–0.05] | <0.001 | 0.07[0.05–0.11] | <0.001 | 0.06[0.04–0.10] | <0.001 | |||||||
| <0.001 | <0.001 | <0.001 | ||||||||||||
| Initial symptoms | ||||||||||||||
| Isolated dysfunction of long tracts | 964 | – | Reference | – | – | – | – | |||||||
| Isolated optic neuritis | 335 | 0.76[0.63–0.91] | 0.004 | – | – | – | – | |||||||
| Isolated brainstem dysfunction | 159 | 0.96[0.75–1.22] | 0.72 | – | – | – | – | |||||||
| Combination of symptoms | 386 | 1.03[0.88–1.22] | 0.71 | – | – | – | – | |||||||
| 0.02 | ||||||||||||||
Data on the patients who did not reach an end point were censored at the time of the last clinic visit.
The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke, 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. Disability was defined as irreversible when a patient had had a score of 4 or more for at least 6 months, excluding any transient worsening of disability related to relapses.
A hazard ratio below 1 denotes an older age at disability milestones than the reference group whereas a hazard ratio over 1 denotes a younger age at disability milestones.
Final Cox regression models of the age of the patients at the time of assignment of an irreversible disability score of DSS 4, DSS 6, and DSS 7 among 1844 patients with multiple sclerosis
| Variable | No. of patients (N = 1844) | Age at assignment of DSS 4* | Age at assignment of DSS 6* | Age at assignment of DSS 7* | ||||||||||
| Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | |||||||||
| Initial course of multiple sclerosis | ||||||||||||||
| Exacerbating-remitting | 1562 | – | Reference | – | Reference | – | Reference | |||||||
| Progressive | 282 | 3.89 | <0.001 | 2.72 | <0.001 | 2.39 | <0.001 | |||||||
| [3.25–4.65] | [2.21–3.35] | [1.84–3.10] | ||||||||||||
| Gender | ||||||||||||||
| Male | 657 | – | Reference | – | Reference | – | Reference | |||||||
| Female | 1187 | 0.85[0.75–0.96] | 0.01 | 0.75[0.63–0.88] | 0.001 | 0.74[0.60–0.91] | 0.001 | |||||||
| Age at onset of multiple sclerosis | ||||||||||||||
| 0–19 years | 216 | – | Reference | – | Reference | – | Reference | |||||||
| 20–29 years | 690 | 0.54[0.43–0.67] | <0.001 | 0.55[0.42–0.73] | <0.001 | 0.49[0.35–0.69] | <0.001 | |||||||
| 30–39 years | 558 | 0.23[0.18–0.28] | <0.001 | 0.29[0.22–0.39] | <0.001 | 0.24[0.17–0.34] | <0.001 | |||||||
| 40–49 years | 272 | 0.10[0.08–0.13] | <0.001 | 0.13[0.10–0.19] | <0.001 | 0.10[0.07–0.16] | <0.001 | |||||||
| More than 50 years | 108 | 0.04[0.03–0.05] | <0.001 | 0.07[0.05–0.11] | <0.001 | 0.06[0.04–0.10] | <0.001 | |||||||
| <0.001 | <0.001 | <0.001 | ||||||||||||
| Initial symptoms | ||||||||||||||
| Isolated dysfunction of long tracts | 964 | – | Reference | – | – | – | – | |||||||
| Isolated optic neuritis | 335 | 0.76[0.63–0.91] | 0.004 | – | – | – | – | |||||||
| Isolated brainstem dysfunction | 159 | 0.96[0.75–1.22] | 0.72 | – | – | – | – | |||||||
| Combination of symptoms | 386 | 1.03[0.88–1.22] | 0.71 | – | – | – | – | |||||||
| 0.02 | ||||||||||||||
| Variable | No. of patients (N = 1844) | Age at assignment of DSS 4* | Age at assignment of DSS 6* | Age at assignment of DSS 7* | ||||||||||
| Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | Hazard ratio**[95% CI] | P-value | |||||||||
| Initial course of multiple sclerosis | ||||||||||||||
| Exacerbating-remitting | 1562 | – | Reference | – | Reference | – | Reference | |||||||
| Progressive | 282 | 3.89 | <0.001 | 2.72 | <0.001 | 2.39 | <0.001 | |||||||
| [3.25–4.65] | [2.21–3.35] | [1.84–3.10] | ||||||||||||
| Gender | ||||||||||||||
| Male | 657 | – | Reference | – | Reference | – | Reference | |||||||
| Female | 1187 | 0.85[0.75–0.96] | 0.01 | 0.75[0.63–0.88] | 0.001 | 0.74[0.60–0.91] | 0.001 | |||||||
| Age at onset of multiple sclerosis | ||||||||||||||
| 0–19 years | 216 | – | Reference | – | Reference | – | Reference | |||||||
| 20–29 years | 690 | 0.54[0.43–0.67] | <0.001 | 0.55[0.42–0.73] | <0.001 | 0.49[0.35–0.69] | <0.001 | |||||||
| 30–39 years | 558 | 0.23[0.18–0.28] | <0.001 | 0.29[0.22–0.39] | <0.001 | 0.24[0.17–0.34] | <0.001 | |||||||
| 40–49 years | 272 | 0.10[0.08–0.13] | <0.001 | 0.13[0.10–0.19] | <0.001 | 0.10[0.07–0.16] | <0.001 | |||||||
| More than 50 years | 108 | 0.04[0.03–0.05] | <0.001 | 0.07[0.05–0.11] | <0.001 | 0.06[0.04–0.10] | <0.001 | |||||||
| <0.001 | <0.001 | <0.001 | ||||||||||||
| Initial symptoms | ||||||||||||||
| Isolated dysfunction of long tracts | 964 | – | Reference | – | – | – | – | |||||||
| Isolated optic neuritis | 335 | 0.76[0.63–0.91] | 0.004 | – | – | – | – | |||||||
| Isolated brainstem dysfunction | 159 | 0.96[0.75–1.22] | 0.72 | – | – | – | – | |||||||
| Combination of symptoms | 386 | 1.03[0.88–1.22] | 0.71 | – | – | – | – | |||||||
| 0.02 | ||||||||||||||
Data on the patients who did not reach an end point were censored at the time of the last clinic visit.
The Kurtzke Disability Status Scale was used to determine the extent of disability (Kurtzke, 1961, 1983). On this scale, a score of 4 indicates limited walking ability but able to walk without aid or rest for more than 500 m, a score of 6 indicates the ability to walk with unilateral support for no more than 100 m without rest, and a score of 7 indicates the ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. Disability was defined as irreversible when a patient had had a score of 4 or more for at least 6 months, excluding any transient worsening of disability related to relapses.
A hazard ratio below 1 denotes an older age at disability milestones than the reference group whereas a hazard ratio over 1 denotes a younger age at disability milestones.
Discussion
In this observational study of the natural history of multiple sclerosis, median ages at assignment of irreversible disability scores of DSS 4, DSS 6 and DSS 7 were estimated by the Kaplan–Meier technique at 44, 55 and 63 years, respectively. The initial course of multiple sclerosis had a statistically significant influence on ages at assignment of DSS 4 and DSS 6. Patients with an exacerbating-remitting initial course indeed reached these landmarks at an older age than patients with a progressive initial course. However, the differences were only 2.7 and 2.3 years for median ages at assignment of DSS 4 and DSS 6, respectively, for a disease usually encompassing several decades of life. For both assignments, there was also an overlap in the 95% CI of these medians. There was no difference when the two groups of patients were compared with respect to assignment of DSS 7. One should notice that patients with a secondary-progressive course of multiple sclerosis were younger at time of assignment of DSS 4, DSS 6 and DSS 7 than those with a course progressive from onset. Conversion from the initial relapsing-remitting phase to the secondary progression, as estimated by the Kaplan–Meier technique, occurs at a rather constant rate of 2.5% of patients converting per year. This rate remains quite stable throughout the course of the disease. The median survival time for the conversion is 19.1 years (Vukusic and Confavreux, 2003). Considering therefore a cohort of patients with multiple sclerosis studied at a given time, some of them not yet in secondary progression, the patients who already have experienced a secondary-progressive course at the time of the survey are distinct: they represent a subgroup of more rapidly worsening forms of multiple sclerosis within the entire group of patients with an exacerbating-remitting initial course. Furthermore, the difference in the percentage of censored patients among exacerbating-remitting and progressive cases, which is greater for the lower disability scores (DSS 4 and DSS 6) with a higher censoring for cases with an exacerbating-remitting initial course, can contribute to the difference in the estimates. Indeed, censoring usually leads to overestimating the medians. It can thus be presumed that the actual age at assignment of irreversible disability levels of patients with an exacerbating-remitting initial course lies somewhere in-between the ages we found for this whole group of patients and the ages we found for the subgroup of patients with a secondary-progressive course, that is closer to the ages we found for patients with a course progressive from onset. Concretely, it can be considered that the initial course of the disease, whether exacerbating-remitting or progressive, does not substantially influence age at disability milestones in multiple sclerosis.
Prevalence of multiple sclerosis in the Rhône-Alpes Region has been estimated to circa 50 per 100 000 inhabitants according to the most recent regional epidemiological study performed (Confavreux et al., 1987). More recently, a nationwide survey of the registries of the ‘Mutualité Sociale Agricole’, which serves as a mandatory and exclusive health insurance system for the rural population in France, led to a prevalence estimate for multiple sclerosis of 71 per 100 000 inhabitants in the Rhône-Alpes Region by January 1, 2003 (Van Bockstael et al., 2004). The Lyon Multiple Sclerosis Cohort strictly speaking is not a population-based but essentially a clinic-based cohort. However, considering the number of patients with multiple sclerosis enrolled in the cohort and the number of patients that could be expected according to the local prevalence studies, one can reasonably admit that our cohort is representative of the population of patients with multiple sclerosis in the area. About half of the patients in the cohort received immunosuppressive drugs, mostly azathioprine, at some point during their disease, mainly during the relapsing-remitting phase, and not before the third relapse. None of these drugs have proven to reduce progression of irreversible disability in multiple sclerosis and therefore should not have biased the chosen end-point measures in our study (Rudick et al., 1997; Noseworthy et al., 2000; Compston and Coles, 2002). Betaseron®, the first disease-modifying agent approved for multiple sclerosis which became available in France in February 1996, has unlikely biased the results of this study, as the database was locked in April 1997.
The demographic and disease-related characteristics of the 1844 patients of our cohort are consistent with those from representative series in the literature (Müller, 1949, 1951; McAlpine and Compston, 1952; Leibowitz et al., 1964a, b; Kurtzke et al., 1968, 1970, 1973, 1977; Panelius, 1969; Leibowitz and Alter, 1970, 1973; Poser, 1978; Fog and Linneman, 1970; Broman et al., 1981; Poser et al., 1982; Clark et al., 1982; Detels et al., 1982; Visscher et al., 1984; Phadke, 1990; Minderhoud et al., 1988; Weinshenker et al., 1989a, b, 1991; Miller et al., 1992; Riise et al., 1992; Runmarker and Andersen, 1993; Trojano et al., 1995; Kantarci et al., 1998; Cottrell et al., 1999; Kremenchutzky et al., 1999; Amato and Ponziani, 2000; Myhr et al., 2001; Eriksson et al., 2003). They have already been described in detail (Confavreux et al., 2000, 2003). The median time from onset of multiple sclerosis to the initial visit in our clinic was 3 years, with a range from 0 to 53 years. This being said, a specific effort is always made to obtain data from the original medical files, especially for the first neurological episode, and on the clinical course and disability until the initial clinic visit. This effort is facilitated by the existing regional network of neurologists in our area. Most of them have been trained in our hospital in Lyon and maintain tight connections with our clinic for their patients suffering from multiple sclerosis. This organization helps updating regularly the database with follow-up data once the patient has been registered in the database. Except for very few cases, the results of the neurological examinations registered in the database are those of the neurological examinations performed in the clinic.
By definition, the date of onset of progression is assessed in retrospect, once the required six-month duration of continuous neurological worsening has been confirmed. Therefore, there is always some uncertainty regarding this parameter. However, in the collaborative multicentre EVALUED study using the EDMUS system (Confavreux et al., 1992) which involved the Lyon clinic and five other European centres—with 180 patients with multiple sclerosis in all, that is to say two examiners and 30 patients for each centre—the inter-examiner reliability was almost perfect with a κ-value of 0.92 when cases had to be categorized according to an exacerbating remitting or progressive onset (Amato et al., 2004). When both examiners had to decide on the development of secondary progression, agreement was again substantial with a κ-value of 0.76. When they had to date the onset of secondary progression, agreement between both examiners was reached with ≤1 year difference in 72% of cases. Concerning the Kurtzke Disability Status Scale, both examiners reached the same score or with ≤1.0 point difference in 78 and 97% of the examinations, respectively (Amato et al., 2004).
The originality of our study lies in its being the first ever to show that age at assignment of disability landmarks is not substantially influenced by the type of the initial course of multiple sclerosis, be it exacerbating-remitting or progressive. This is further evidence that neurological relapses, whenever they may occur, have only a limited influence on the accumulation of irreversible disability in the long-term (Confavreux et al., 2000, 2003). This age-dependency of the accumulation of irreversible disability, whatever the initial course of the disease, finds preliminary support in brain imaging studies of patients with multiple sclerosis (Filippi et al., 2001; Kassubek et al., 2003) and in clinico-pathological studies of experimental models of the disease (Smith et al., 1999). However, it would be an oversimplification to consider that accumulation of irreversible disability in multiple sclerosis is strictly age-dependent. As shown in this paper, age at clinical onset of multiple sclerosis is also influential: the younger the onset, the younger the age at assignment of disability milestones. Similarly, the younger the onset, the longer the survival time for converting from an exacerbating-remitting onset of the disease to secondary progression (Vukusic and Confavreux, 2003) and therefore the lower the rate of patients converting per year (S Vulusic and C Confavreux, unpublished personal data). This confirms the complex interaction existing between age at clinical onset of multiple sclerosis and current age, which has already been observed in the Lyon cohort (Confavreux, 1977; Confavreux et al., 1980) and in an Italian study (Trojano et al., 2002). Furthermore, gender is also influential, females reaching disability milestones at an older age than males. By contrast, initial symptomatology of multiple sclerosis was essentially not influential in our series.
The statistical results we have obtained at the level of a large and representative population of patients with multiple sclerosis depict a very homogeneous prognosis of the disease. However, it is important to realize that this does not preclude the considerable inter-individual variability in age at disability milestones in multiple sclerosis. This can be observed in Table 1 by comparing the 25th and 75th percentiles to the median age at disability steps. The age-dependency phenomenon described here surmounts this however, with an absence of influence of the type of the initial course of the disease on the age at disability milestones.
At the pathophysiological level, these clinical results suggest that acute multifocal recurrent inflammation, namely the substratum of clinically detectable relapses (Youl et al., 1991), has only a limited effect on the early diffuse chronic neurodegeneration, which is likely to be the substratum of clinical progression in multiple sclerosis (Confavreux and Vukusic, 2002). They also suggest that, at least to some extent, ageing-related processes might play a role in the chronic diffuse neurodegeneration of multiple sclerosis. Physiological age-related decrease in remyelinating capacity, immunological reactivity or adaptative response to oxidative stress are well-demonstrated (Sohal and Weindruch, 1996; Sim et al., 2002; Chari et al., 2003; Fraker and Lill-Elghanian, 2004). It may therefore be hypothesized that chronic inflammation in multiple sclerosis could elicit an accelerated ageing of the central nervous system. This suggests that, apart from acute focal recurrent inflammation and diffuse chronic neurodegeneration, accelerated ageing-related mechanisms might be taken into account in therapeutic strategies for the control of multiple sclerosis.
This work was supported by contracts with the Commission of the European Communities Directorate General XII (BMH1-CT93-1529, CIPD-CT94-0227 and BMH4-CT96-0064), by funds from the Ligue Française contre la Sclérose En Plaques (L.F.S.E.P.), the Association pour la Recherche sur la Sclérose en Plaques (A.R.S.E.P.), the Association des Sclérosés En Plaques Loire Sud (ASEPLS), the Groupe Bouygues Telecom, the Compagnons du Beaujolais, the Lions Club ‘Villages beaujolais’, and by unconditional grants from Biogenidec France, Schering SA, Serono France, and Teva Pharma France. We are indebted to Prof. Patrice Adeleine for contributing to the statistical analysis; to the patients for taking part in the Lyon Multiple Sclerosis Database; Profs Brian Weinshenker for reviewing an earlier draft of the manuscript; Mrs Marie-Françoise Belin, Prof Jacques Estève and Dr Serge Nataf for their helpful discussions about the paper; Drs Gilbert Aimard, Michel Devic, Thibault Moreau, Jean-Jacques Ventre, Jean-François de Saint Victor, Iuliana Achiti, Sandrine Blanc, Françoise Bouhour, Patricia Cortinovis-Tourniaire, Françoise Durand-Dubief, Laurence Gignoux, Jérôme Grimaud, Romain Marignier, Christel Renoux, and Georges Riche for their contribution to the development of the database; Mr Albert Biron, Dr Bernard Frangoulis and Mrs Catherine Vidal for maintaining the database; and Mrs France-Isabelle Pairel for secretarial assistance.
References
Amato MP, Ponziani G. A prospective study on the prognosis of multiple sclerosis.
Amato MP, Grimaud J, Achiti I, Bartolozzi ML, Adeleine P, Hartung HP, et al. European validation of a standardized clinical description of multiple sclerosis.
Broman T, Andersen O, Bergmann L. Clinical studies on multiple sclerosis. I. Presentation of an incidence material from Gothenburg.
Chari DM, Crang AJ, Blakemore WF. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age.
Clark VA, Detels R, Visscher BR, Valdiviezo NL, Malmgren RM, Dudley JP. Factors associated with a malignant or benign course of multiple sclerosis.
Confavreux C. L'histoire naturelle de la sclérose en plaques. Etude par informatique de 349 observations. Thèse de Médecine
Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients.
Confavreux C, Darchy P, Alperovitch A, Aimard G, Devic M. Le sud-Est français, zone ‘à haut risque’ de Sclérose en Plaques?
Confavreux C, Compston DAS, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European Database for Multiple Sclerosis.
Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis.
Confavreux C, Vukusic S. Natural history of multiple sclerosis: implications for counseling and therapy.
Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process.
Cottrell DA, Kremenchutzky M, Rice GPA, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis.
Detels R, Clark VA, Valdiviezo NL, Visscher BR, Malmgren RM, Dudley JP, et al. Factors associated with a rapid course of multiple sclerosis.
Eriksson M, Andersen O, Runmarker B. Long-term follow-up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis.
Filippi M, Wolinsky JS, Sormani MP, Comi G; European/Canadian Glatiramer Acetate Study Group. Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis.
Fog T, Linneman F. The course of multiple sclerosis in 73 cases with computer designed curves.
Fraker PJ, Lill-Elghanian DA. The many roles of apoptosis in immunity as modified by aging and nutritional status.
Kantarci O, Siva A, Eraksoy M, Karabudak R, Sutlas N, Agaoglu J, et al. and the Turkish Multiple Sclerosis Study Group (TUMSSG). Survival and predictors of disability in Turkish MS patients.
Kassubek J, Tumani H, Ecker D, Kurt A, Ludolph AC, Juengling FD. Age-related brain parenchymal fraction is significantly decreased in young multiple sclerosis patients: a quantitative MRI study.
Kremenchutzky M, Cottrell D, Rice G, Hader W, Baskerville J, Koopman W, et al. The natural history of multiple sclerosis: a geographically based study. 7. Progressive-relapsing and relapsing-progressive multiple sclerosis: a re-evaluation.
Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS).
Kurtzke JF, Beebe GW, Nagler B, Auth TL, Kurland LT, Nefzger MD. Studies on natural history of multiple sclerosis. 4: Clinical features of the onset bout.
Kurtzke JF, Beebe GW, Nagler B, Nefzger MD, Auth TL, Kurland LT. Studies on the natural history of multiple sclerosis. V. Long-term survival in young men.
Kurtzke JF, Beebe GW, Nagler B, Auth TL, Kurland LT, Nefzger MD. Studies on the natural history of multiple sclerosis. 7: Correlates of clinical changes in an early bout.
Kurtzke JF, Beebe GW, Nagler B, Kurland LT, Auth TL. Studies on the natural history of multiple sclerosis—Early prognostic features of the later course of the illness.
Leibowitz U, Alter M, Halpern L. Clinical studies of multiple sclerosis in Israel. III. Clinical course and prognosis related to age at onset.
Leibowitz U, Halpern L, Alter M. Clinical studies of multiple sclerosis in Israel. I. A clinical analysis based on a country-wide survey.
Leibowitz U, Alter M. Clinical factors associated with increased disability in multiple sclerosis.
Leibowitz U, Alter M. Multiple sclerosis: clues to its cause. Amsterdam and London: North-Holland Publishing Company;
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey.
McAlpine D, Compston N. Some aspects of the natural history of disseminated sclerosis: incidence, course and prognosis; factors affecting onset and course.
Miller DH, Hornabrook RW, Purdie G. The natural history of multiple sclerosis: a regional study with some longitudinal data.
Minderhoud JM, Van der Hoeven JH, Prange AJ. Course and prognosis of chronic progressive multiple sclerosis. Results of an epidemiological study.
Müller R. Studies on disseminated sclerosis. With special reference to symptomatology, course and prognosis.
Müller R. Course and prognosis of disseminated sclerosis in relation to age at onset.
Myhr KM, Riise T, Vedeler C, Nortvedt MW, Gronning R, Midgard R, et al. Disability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pension.
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis.
Panelius M. Studies on epidemiological, clinical and etiological aspects of multiple sclerosis.
Phadke JG. Clinical aspects of multiple sclerosis in north-east Scotland with particular reference to its course and prognosis.
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols.
Poser S, Raun NE, Poser W. Age at onset, initial symptomatology and the course of multiple sclerosis.
Poser S. Multiple sclerosis. An analysis of 812 cases by means of electronic data processing. Berlin: Springer-Verlag;
Riise T, Gronning M, Fernandez O, Lauer K, Midgard R, Minderhoud JM, et al. Early prognostic factors for disability in multiple sclerosis, a European multicenter study.
Rudick R, Cohen JA, Weinstock-Guttman B, Kinkel RP, Ransohoff RM. Management of multiple sclerosis.
Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up.
Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in MS: report by the panel on the evaluation of experimental trials of therapy in MS.
Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation.
Smith ME, Eller NL, McFarland HF, Racke MK, Raine CS. Age dependence of clinical and pathological manifestations of autoimmune demyelination. Implications for multiple sclerosis.
Trojano M, Avolio C, Manzari C, Calo A, De Robertis F, Serio G, et al. Multivariate analysis of predictive factors of multiple sclerosis course with a validated method to assess clinical events.
Trojano M, Liguori M, Bosco Zimatore G, Zimatore G, Bugarini R, Avolio C, et al. Age-related disability in multiple sclerosis.
Van Bockstael V, Gosselin S, Vukusic S, Confavreux C. Prevalence of multiple sclerosis in French farmers: a nationwide survey.
Visscher BR, Liu KS, Clark VA, Detels R, Malmgren RM, Dudley JP. Onset symptoms as predictors of mortality and disability in multiple sclerosis.
Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis.
Weinshenker BG, Bass B, Rice GPA, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability.
Weinshenker BG, Bass B, Rice GPA, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. II. Predictive value of the early clinical course.
Weinshenker BG, Rice GPA, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 3. Multivariate analysis of predictive factors and models of outcome.