-
PDF
- Split View
-
Views
-
Cite
Cite
Marco van Eijk, Cindy P. A. A. van Roomen, G. Herma Renkema, Anton P. Bussink, Laura Andrews, Edward F. C. Blommaart, Alan Sugar, Arthur J. Verhoeven, Rolf G. Boot, Johannes M. F. G. Aerts, Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity, International Immunology, Volume 17, Issue 11, November 2005, Pages 1505–1512, https://doi.org/10.1093/intimm/dxh328
Close - Share Icon Share
Abstract
Man has been found to produce highly conserved chitinases. The most prominent is the phagocyte-derived chitotriosidase, the plasma levels of which are markedly elevated in some pathological conditions. Here, we report that both polymorphonuclear neutrophils (PMNs) and macrophages (mϕ) are a source of chitotriosidase. The enzyme is located in specific granules of human PMNs and secreted following stimulation with granulocyte macrophage colony-stimulating factor (GM-CSF). In addition, GM-CSF induces expression of chitotriosidase in mϕ that constitutively secrete the enzyme and partly accumulate it in their lysosomes. Studies with recombinant human chitotriosidase revealed that the enzyme targets chitin-containing fungi. These findings are consistent with earlier observations concerning anti-fungal activity of homologous plant chitinases and beneficial effects of GM-CSF administration in individuals suffering from invasive fungal infections. In conclusion, chitotriosidase should be viewed as a component of the innate immunity that may play a role in defence against chitin-containing pathogens and the expression and release of which by human phagocytes is highly regulated.
Introduction
Chitotriosidase was first discovered in plasma of patients suffering from Gaucher disease. It was found that the 1000-fold-elevated enzyme originates from lipid-laden macrophages (mϕ) that accumulate in various tissues of Gaucher patients (1). Chitotriosidase has subsequently been purified from the spleen of a Gaucher patient and its cDNA was cloned from a human mϕ cDNA library (2, 3). Chitotriosidase is known to exist in two forms, a 50-kDa protein and a 39-kDa enzyme that is produced thereof by proteolytic processing. The 50-kDa protein consists of a C-terminal chitin-binding domain, a hinge region and the 39 kDa N-terminal domain that has chitinase activity (4). In lysosomes of mϕ, processing of 50-kDa chitotriosidase to the 39-kDa form occurs. In circulation exclusively the 50-kDa enzyme is present. A 24-bp insertion in exon 10 of the chitotriosidase gene that prevents formation of active enzyme is occurring pan ethnically (5). In most ethnic groups, ∼5% of all individuals are homozygous for this mutation and consequently lack chitotriosidase. A lower frequency of the deficiency occurs in black populations. Chitotriosidase belongs to the family 18 of glycosyl hydrolases and is an endoglucosaminidase that cleaves and shows transglycosylation activity towards chitin, the linear polymer of N-acetyl-D-glucosamine present in coatings of many pathogens such as protozoan parasites, fungi and nematodes (4, 6, 7). Homologous chitinases are expressed in several organisms, including plants, in which they are thought to play a role in innate immunity and in particular in defence against fungal pathogens. These findings suggest that chitotriosidase may play a comparable role in man. We, therefore, have studied the potential role of chitotriosidase in innate immunity by investigating its cellular production and localization, its release into the circulation and the effects of recombinant human chitotriosidase on chitin-containing fungi. We here report that chitotriosidase indeed inhibits hyphal growth of chitin-containing fungi.
Methods
Cell culture
Whole blood from buffy coats was depleted for erythrocytes using ammonium chloride (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and this fraction was referred to as total blood cells depleted of erythrocytes. PBMCs were isolated by density centrifugation using Lymphoprep (Nycomed, Roskilde, Denmark). Monocytes were isolated from PBMCs by enrichment on a percoll gradient (Amersham Biosciences, Piscataway, USA), consisting of 34, 45 and 60% layers. Monocytes were taken from the 34–45% interface, washed in PBS, followed by a 1-h adherence step at 37°C. Non-adhering cells were removed and further maturation towards mϕ was achieved by continued adherence to plastic in 12-well culture plates (Corning Incorporated, NY, USA) in RPMI 1640 (BioWhittaker, Verviers, Belgium) and 10% human serum (BioWhittaker) to allow induction of chitotriosidase. Monocytes were also matured in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF; 50 ng ml−1), macrophage colony-stimulating factor (M-CSF; 10 ng ml−1), IL-4 (40 ng ml−1) or IFN-γ (10 ng ml−1; all from Peprotech, London, UK). Phenotypical maturation was confirmed by light microscopy analysis and analysis of cell-surface marker expression patterns by FACS. Following 8 days of culture, chitotriosidase expression levels were determined by western blot analysis and enzyme activity was determined in cell-free culture supernatants.
Polymorphonuclear neutrophils (PMNs) were isolated from lymphoprep cell pellets which were depleted for erythrocytes on a 1% dextran 500/HBSS (BioWhittaker) gradient followed by 10 min erythrocyte lysis with ammonium chloride. PMNs were taken up in RPMI/0.5% BSA and seeded at 4 million cells in a final volume of 200 μl. PMNs were stimulated with GM-CSF (50 ng ml−1), N-formyl-methionyl-leucyl-phenylalanine (fMLP; 1 μM), platelet-activating factor (PAF; 1 μM) and cytochalasin B (5 μg ml−1; all Sigma, MO, USA). Following 10–60 min of incubation, cell-free supernatants were analysed for enzyme activity.
Cryoultramicrotomy and immunolabelling
Leukocytes from peripheral blood were fixed with a mixture of 0.5% (v/v) glutaraldehyde and 4% (w/v) PFA and pelleted in 10% (w/v) gelatine in PBS. Ultra-thin frozen sections were incubated at room temperature with rabbit anti-lactoferrin antiserum (1:400 dilution; Cappel Laboratories, Malvern, USA) followed by incubation with 5 nm gold-conjugated goat anti-rabbit IgG, then treated with 1% glutaraldehyde for 10 min to prevent any interference between the different antibody gold complexes (Amersham Biosciences) on the sections and finally incubated with rabbit anti-chitotriosidase antiserum (1:100 dilution) (4) and anti-rabbit IgG linked to 10-nm gold particles. For controls, primary antibody was replaced by non-relevant rabbit antiserum. After immunolabelling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined under a Philips CM10 electron microscope.
Enzyme activity assay and western blot
Chitotriosidase enzyme activity was determined in cell-free supernatants of stimulated neutrophils, in cell-free culture supernatants of stimulated mϕ and in lysates (prepared in PBS/0.1% Triton X-100 and sonicated for three cycles of 5 s) of non-stimulated PBMCs, non-stimulated PMNs and non-stimulated total blood cells depleted of erythrocytes using the fluorescent substrate 4-methyl umbeliferyl (4-MU) β-D-N,N′,N″-(MU)-triacylchitotriose (2). For β-hexosaminidase activity, N-acetylglucosaminide 4-MU was used. Protein concentrations were determined according to the Bradford method (8). When culture supernatants were analysed, enzyme activities were calculated and expressed as nanomoles per millilitre per hour. Cell lysate activities were expressed as nanomoles per millilitre per hour. For mϕ culture supernatants nanomoles per millilitre per hour activities were calculated first and subsequently activities were expressed on the y-axis as fold induction compared with culture medium alone. Statistical analysis was performed using the Student's t-test; P <0.05 was considered significant.
For western blot analysis, cell lysates of non-stimulated total blood cells depleted of erythrocytes, non-stimulated PBMCs, non-stimulated PMNs, monocytes and matured mϕ (matured in culture medium alone or stimulated with GM-CSF, IL-4, IFN-γ or M-CSF) were prepared in RIPA (150 mM NaCl, 10 mM Tris pH 7.2, 0.1% SDS, 1% Triton, 1% deoxycholate, 5 mM EDTA) buffer and lysates representing equal amounts of protein were separated on an 8% SDSPAGE gel, transferred to nitrocellulose (Fischer Scientific, Pittsburgh, USA), probed using rabbit anti-chitotriosidase polyclonal (4) or mouse anti-β-actin mAbs (Sigma) to verify equal sample loading and stained with specific HRP conjugates.
Recombinant human chitotriosidase
Recombinant human chitotriosidase was expressed in mouse C127i cells using a bovine papilloma virus-based vector (9). Following filtration and concentration, chitotriosidase was purified on an ANX Sepharose FF column (Pharmacia, Uppsala, Sweden). The flow through and wash fraction were pooled, adjusted to pH = 5 and subsequently applied to a cation exchange column (Pharmacia).
In vitro studies
In vitro anti-fungal activity was assessed in several assays. The agar-diffusion assay was performed according to the method described earlier (10). Morphological changes were monitored by light microscopy. Hypotonic medium was applied to Mucor rouxii hyphae upon chitotriosidase or vehicle incubation and tip lysis was monitored. The minimal inhibitory concentration of recombinant human chitotriosidase was determined by the broth microdilution assay in accordance with the National Committee For Clinical Laboratory Standards M27-P to address anti-fungal efficacy against isolates of Cryptococcus neoformans at concentrations ranging from 128 to 0.25 μg ml−1. Inhibition was evaluated after 48 h. The hyphae formation assay was carried out according to the following procedure; recombinant human chitotriosidase was added to wells containing 1 × 106Candida albicans (strain #64, from A.S.). The plate was incubated at 37°C for 98 h and then switched to room temperature for 24 h to allow the formation of hyphae.
In vivo studies
ICR male mice were purchased from Taconic Laboratories (Germantown, USA) and used for all models of systemic fungal infections. Candida albicans (strain #64 from A.S.) were prepared in PBS at 1 × 107 organisms ml−1. Aspergillus fumigatus (strain #H5978864, from A.S.) spores were suspended in saline at 5 × 105 organisms ml−1. Mice were rendered neutropenic with 5-Fluorouracil (150 mg kg−1; Sigma), which was administered intravenously For Candidiasis studies cyclophosphamide (Sigma) was used at 30 mg kg−1, for Aspergillosis at 200 mg kg−1. Cyclophosphamide was administered daily intraperitoneally (i.p.). All infections were administered as a bolus tail vein injection. Lyophilized recombinant human chitotriosidase was reconstituted in sterile PBS to a final concentration of 20 mg ml−1. Twenty-four hours following infection, animals were dosed with an i.p. injection of recombinant human chitotriosidase. The number of animals alive in each group was scored daily. Percent survival was calculated by dividing the number of living mice by the number of total mice in each group. Healthy individuals were injected with GM-CSF as described earlier (11). PMN counts, lactoferrin levels and chitotriosidase activity were measured in plasma samples, drawn after indicated time points. Lactoferrin levels were determined by radioimmune assay, according to the method described by Nuijens et al. (12). All animal and human studies were approved for by appropriate review boards. Informed consent was provided according to the Declaration of Helsinki.
Results
Chitotriosidase expression and secretion by human phagocytes
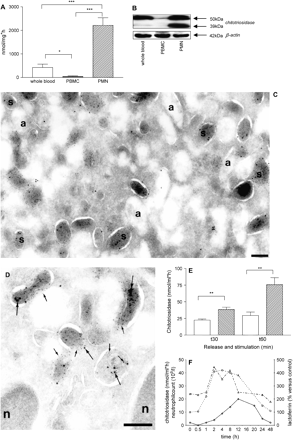
Human PMNs, but not lymphocytes and monocytes, are a major source of chitotriosidase in blood of healthy individuals. This is indicated by analysis of both enzyme activity and protein levels in non-stimulated total blood cells depleted of erythrocytes, PBMCs and PMNs (Fig. 1A, B). The exact sub-cellular location of the enzyme in human PMNs was studied. For this purpose, PMNs were treated with different combinations of stimuli to obtain differential de-granulation of intracellular granules (13). Table 1 shows that exposure of purified PMNs to a condition that induces secretion of secretory vesicles (i.e. incubation with PAF) does not result in the release of chitotriosidase. Exposure of PMNs to PAF + fMLP, which also induces the release of components of specific granules, results in almost complete secretion of chitotriosidase. Finally, exposure to cytochalasin B + fMLP, which causes the secretion of proteins from all vesicles including azurophilic granules, does not lead to substantially more secretion of chitotriosidase. This is in contrast to the increased secretion of β-hexosaminidase, a lysosomal enzyme present in azurophilic granules of PMNs. These results suggest that in PMNs chitotriosidase is not present in the lysosome-like azurophilic granules, but in the specific granules. Using immunogold double-labelling experiments, chitotriosidase was indeed only detected in lactoferrin-containing compartments, the specific granules (Fig. 1C, D) (10, 11). No double staining for the azurophilic marker myeloperoxidase and chitotriosidase was observed (data not shown).
Chitotriosidase is expressed in specific granules of human PMNs and can be released by GM-CSF. (A) Non-stimulated PMNs when compared with non-stimulated total blood depleted of erythrocytes and PBMCs show increased chitotriosidase activity in PBS/0.1% Triton X-100 cell lysates (B) and increased chitotriosidase expression as addressed by western blot analysis for chitotriosidase (39 and 50 kDa) and β-actin (42 kDa) when compared with whole blood depleted of erythrocytes and PBMCs. Micrographs showing immuno double labelling of PMNs for chitotriosidase and lactoferrin. Panels (C and D): cryosections of PMNs labelled to detect chitotriosidase (10-nm gold particles, large arrows in panel D) and lactoferrin (5-nm gold particles, small arrows in panel D). Chitotriosidase was found in association with the specific granules (s), which were marked with lactoferrin. The almost electron-lucent azurophilic granules (a) were not labelled. Bars, 200 nm. (E) In vitro release of chitotriosidase from PMNs stimulated with GM-CSF (50 ng ml−1). Control: open bars; GM-CSF: hatched bars. (F) In vivo release of chitotriosidase (open triangles), lactoferrin (open circles) and PMN counts (open diamonds) in human subjects after GM-CSF injection. Chitotriosidase activities are indicated as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (n = 5).
Release of chitotriosidase from PMNs
. | β-hexosaminidase activity (nmol ml−1) . | Chitotriosidase activity (nmol ml h−1) . |
|---|---|---|
| None | 76 ± 17 | 7 ± 1 |
| PAF (secretory vesicles) | 163 ± 35 | 15 ± 7 |
| PAF + fMLP (+specific granules) | 197 ± 33 | 244 ± 12 |
| Cytochalasin B + fMLP (+azurophilic vesicles) | 1970 ± 184 | 260 ± 22 |
. | β-hexosaminidase activity (nmol ml−1) . | Chitotriosidase activity (nmol ml h−1) . |
|---|---|---|
| None | 76 ± 17 | 7 ± 1 |
| PAF (secretory vesicles) | 163 ± 35 | 15 ± 7 |
| PAF + fMLP (+specific granules) | 197 ± 33 | 244 ± 12 |
| Cytochalasin B + fMLP (+azurophilic vesicles) | 1970 ± 184 | 260 ± 22 |
Enzyme activities were measured in cell-free supernatants after 10 min stimulation. Mean values ± SD of three donors are depicted.
Release of chitotriosidase from PMNs
. | β-hexosaminidase activity (nmol ml−1) . | Chitotriosidase activity (nmol ml h−1) . |
|---|---|---|
| None | 76 ± 17 | 7 ± 1 |
| PAF (secretory vesicles) | 163 ± 35 | 15 ± 7 |
| PAF + fMLP (+specific granules) | 197 ± 33 | 244 ± 12 |
| Cytochalasin B + fMLP (+azurophilic vesicles) | 1970 ± 184 | 260 ± 22 |
. | β-hexosaminidase activity (nmol ml−1) . | Chitotriosidase activity (nmol ml h−1) . |
|---|---|---|
| None | 76 ± 17 | 7 ± 1 |
| PAF (secretory vesicles) | 163 ± 35 | 15 ± 7 |
| PAF + fMLP (+specific granules) | 197 ± 33 | 244 ± 12 |
| Cytochalasin B + fMLP (+azurophilic vesicles) | 1970 ± 184 | 260 ± 22 |
Enzyme activities were measured in cell-free supernatants after 10 min stimulation. Mean values ± SD of three donors are depicted.
Consistent with its location in specific granules, GM-CSF was found to induce release of chitotriosidase from human PMNs in vitro (Fig. 1E). This was further confirmed by analysis of serial plasma samples from healthy individuals to which GM-CSF had been administered (11). Analysis revealed that plasma chitotriosidase and lactoferrin occur in parallel: 2 h after GM-CSF injection both become detectable (Fig. 1F). This is not caused by increased neutrophil cell numbers because these levels peak later in time (∼12 h).
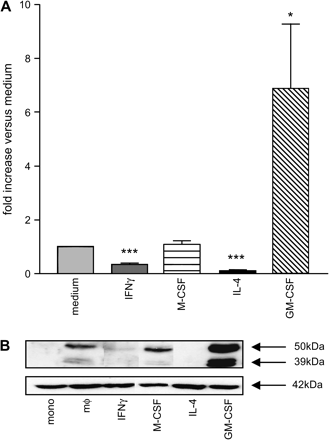
Chitotriosidase, which is absent in monocytes, is expressed by mature human mϕ, being constitutively secreted as 50-kDa protein and present as 39-kDa enzyme in their lysosomes (2, 3). To address which factors drive chitotriosidase expression in mϕ, we have cultured monocytes in culture medium alone or in the presence of either M-CSF, IL-4, IFN-γ or GM-CSF. We first analysed the presence of chitotriosidase activity in culture medium using the fluorescent 4-MU-chitotriose substrate. We observed that GM-CSF super-induces production of chitotriosidase as measured by enzyme activity in the medium (Fig. 2A) or cellular protein levels (Fig. 2B). Consistent increases in chitotriosidase mRNA in cells exposed to GM-CSF were observed (data not shown). In contrast, both IFN-γ (classical activation of mϕ) and IL-4 (alternative activation of mϕ) prevented induction of chitotriosidase. M-CSF exerted little effect on chitotriosidase production. Monocyte-derived dendritic cells generated in the presence of both GM-CSF and IL-4 do not express the chitinase (data not shown).
Regulation of chitotriosidase expression in human mϕ. (A) Chitotriosidase activity in culture supernatants of mϕ matured with medium or cytokines (for details see Methods). Activities are calculated as nanomoles per millilitre per hour and expressed on the y-axis as fold increase compared with stimulation with medium only. Data are indicated as mean ± SEM, and stimulation with medium was compared with cytokine maturation; *P < 0.05 and ***P < 0.001 (n = 8). (B) Western blot analysis of chitotriosidase (39 and 50 kDa) and β-actin (42 kDa) in RIPA cell lysates of cytokine-matured mϕ.
In vitro activity of recombinant human chitotriosidase
Taking into account the phagocyte-specific expression of chitotriosidase, we next addressed its potential role in innate immune responses. Immune-compromised individuals show an increased risk to life-threatening fungal infections and it has been described that following GM-CSF treatment improved anti-fungal responses are observed, supposedly via PMNs and monocytes/mϕ (14, 15). GM-CSF induces chitotriosidase expression in maturing human mϕ and also triggers release of chitotriosidase from human PMNs. It may be therefore speculated that human chitotriosidase, in line with the role of homologous plant chitinases, exerts anti-fungal activity (3, 16). This possibility prompted us to study the potential activity of chitotriosidase towards several chitin-containing fungi. For this purpose, 50-kDa human chitotriosidase was recombinantly produced and purified to homogeneity.
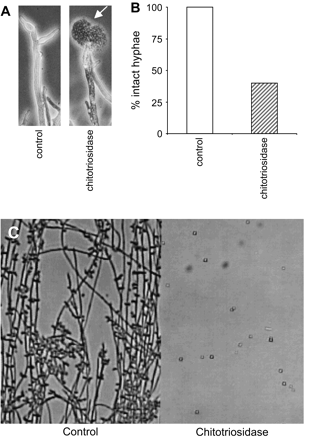
We have earlier shown that recombinant chitotriosidase can degrade cell wall chitin of C. albicans (17). We further studied the possible anti-fungal activity in several in vitro models. In a broth microdilution assay, we observed clear growth inhibition of C. neoformans. The minimal inhibitory concentration of recombinant human chitotriosidase to inhibit growth for 48 h of the fungi was <0.25 μg ml−1. Strikingly, we did find profound effects when fungal morphology was addressed by microscopy. We observed the formation of atypical blebs, followed by hyphal tip bursting when M. rouxii is exposed to chitotriosidase and subsequently placed in a hypotonic environment (Fig. 3A). Further analysis revealed that 60% of hyphae collapsed in the hypotonic environment as a result of chitotriosidase exposure (Fig. 3B). A more dramatic effect was found when we studied the formation of hyphae in cultures of C. albicans. We observed that addition of recombinant human chitotriosidase prevented the transition from the yeast form towards the hyphal form (Fig. 3C).
In vitro anti-fungal activity of recombinant human chitotriosidase. (A) Left panel, control hyphae: right panel, recombinant human chitotriosidase (100 μg ml−1)-induced hyphal tip lysis in Mucor rouxii, see arrow head. (B) Graph of quantification of tip lysis in hypotonic environment after incubation with recombinant human chitotriosidase. (C) Recombinant human chitotriosidase (1 mg ml−1) blocks formation of hyphae in Candida albicans. Control-grown C. albicans (left panel), or in the presence of chitotriosidase (right panel).
In conclusion, in vitro exposure of chitin-containing fungi to recombinant chitotriosidase results in beneficial effects as revealed by growth inhibition, hyphal tip bursting or prevention of hyphal switch.
Recombinant human chitotriosidase improves survival in neutropenic mouse models of Candidiasis and Aspergillosis
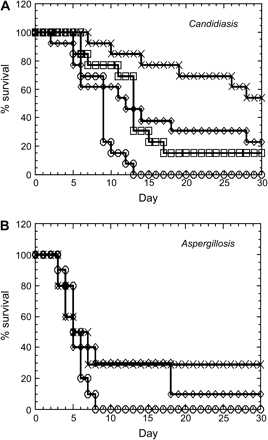
The in vivo efficacy of recombinant human chitotriosidase was studied in neutropenic mouse models of systemic Candidiasis and systemic Aspergillosis, the major causes of mortality in immunosuppressed individuals (14, 15). Recombinant human chitotriosidase, when administrated daily by i.p. injection for 10 consecutive days, promoted survival in a dose-dependent manner when compared with control Candidiasis mice (Fig. 4A). In the control group, all mice died by day 14 of the study. In the groups treated with chitotriosidase, a clear increase in survival was observed. When treated with 10 and 30 mg kg−1, ∼30–40% of the mice survived at this time point, whereas in the group treated with 100 mg kg−1 a striking increase to 80% survival was achieved. The latter group also demonstrates highest survival (55%) at the end-point of the study and this is ∼20% in the lower dose groups. Of note, recombinant chitotriosidase activity rapidly disappeared from the circulation, 90% is lost 1 h post-dose and the tissue half-life is 3 h. Efficacy could be improved by repetitive dosing (data not shown).
Recombinant human chitotriosidase inhibits mortality in mouse models of neutropenic Candidiasis and Aspergillosis. (A) In Candidiasis, three doses of recombinant human chitotriosidase were used and compared with vehicle control. Compared with the control group (open circles) a dose-dependent increase of survival is observed with 10 mg kg−1 (open squares), 30 mg kg−1 (open diamonds) and 100 mg kg−1 (crosses) chitotriosidase. (B) In Aspergillosis, two doses of recombinant human chitotriosidase were used and compared with vehicle control. Compared with the control group (open circles), a dose-dependent increase of survival is observed with 8 mg kg−1 (open diamonds) and 80 mg kg−1 (crosses) chitotriosidase.
A comparable effect, albeit to a lesser extent, was observed in Aspergillosis mice (Fig. 4B). When compared with control mice, increased survival was seen in the presence of chitotriosidase. At day 8 no survivors were present in the control group, whereas 30% survival was observed in the 8 and 80 mg kg−1 groups. At the end-point of the study, the survival went down to 10% in the 8 mg kg−1 group, but remained stable in the 80 mg kg−1 group. In conclusion, recombinant human chitotriosidase clearly improves survival in mouse models of both systemic Candidiasis and systemic Aspergillosis.
Discussion
Our investigation has shed further light on the relationship between chitotriosidase and phagocytes. We observed that the enzyme is selectively expressed and released upon specific stimuli by human PMNs as well as mϕ. In PMNs, chitotriosidase is found to be stored in the specific granules from which it is released by appropriate stimuli. This finding is consistent with the earlier demonstration of chitinase activity in PMNs and its detection in a proteomic survey of released granular proteins (18–20). Interestingly, human cartilage glycoprotein 39 (HCgp39/YKL40), which is homologous to family 18 chitinases but lacks enzyme activity due to a critical amino acid substitution in the active centre, is also present in specific granules of human neutrophils (21). In certain pathological conditions, mϕ massively produce and secrete chitotriosidase, resulting in markedly elevated serum chitotriosidase levels. Over-production of chitotriosidase by mϕ is for instance induced by accumulation of glycosphingolipids, iron or glycogen in lysosomes of mϕ (22–24). Like chitotriosidase, HCgp39 is also secreted by mϕ and furthermore both family 18 chitinases are strongly induced in mϕ present in atheroslerotic plaques (25, 26).
The interaction between GM-CSF and chitotriosidase expression and release by phagocytes is of great interest. Firstly, the cytokine stimulates the synthesis of chitotriosidase in mϕ. This finding is consistent with a recent report in which serial analysis of gene expression revealed that GM-CSF-matured human mϕ expressed increased chitotriosidase transcripts when compared with M-CSF maturation (27). Secondly, GM-CSF promotes the release of chitotriosidase from PMNs via exocytosis of specific granules. In this connection, it is of interest to mention that administration of GM-CSF has been reported to exert beneficial effects in patients with fungal infections (14, 15).
Our study revealed that chitotriosidase exerts activity towards chitin-containing pathogens both in vitro and in vivo. Firstly, chitotriosidase was found to inhibit growth of C. neoformans, to cause hyphal tip lysis in M. rouxii and to prevent the occurrence of hyphal switch in C. albicans. These data strengthen the earlier observed chitinolytic activity towards cell wall chitin of C. albicans (17). Our in vitro observations were extended in neutropenic mouse models of systemic Candidiasis and systemic Aspergillosis, the main causes of mortality in immunocompromised individuals (14, 15). We observed that recombinant human chitotriosidase clearly improved survival in these mouse models. It is of interest to note that chitotriosidase activity has been found to be raised in plasma of neonates upon systemic Candidiasis (28). This observations made with chitotriosidase are not entirely surprising given the well-documented anti-fungal role of chitinases in plants (16). The possible use of recombinant chitotriosidase to treat life-threatening fungal infections may be attractive from a clinical perspective particularly since Gaucher patients seem to tolerate well 1000-fold-elevated serum levels.
In view of our findings, the common deficiency in chitotriosidase is especially intriguing. Common in various ethnic groups is a 24-bp duplication within exon 10 of the chitotriosidase gene. Homozygous individuals for this mutation have no functional chitotriosidase. In most ethnic groups, ∼35% is carrier and 5% is homozygous to the 24-bp duplication (5). Strikingly, in sub-Saharan regions homozygous cannot be found and virtually no carriers could be detected. On one hand, this suggests that the maintenance of functional chitotriosidase in these regions is necessary due to presence of widespread parasitic diseases (29). In support of this view, it has also been reported that infections with Wuchereria bancrofti, which causes human filariasis, are associated with an increased deficiency in chitotriosidase (30). Additional evidence for a role of chitotriosidase during immunological responses is the observation that the enzyme is shortly and acutely up-regulated both at the level of RNA and activity following stimulation with pro-lactin, IFN-γ, tumour necrosis factor α and LPS, but not with IL-10 (31, 32). Here we showed that not only IFN-γ but also IL-4 prevented induction of the enzyme in maturing mϕ. In addition, we found that enzyme expression is inhibited in mature chitinase expressing mϕ after prolonged, i.e. 24 and 48 h, stimulation with IFN-γ or IL-4 (unpublished results).
On the other hand, the discovery of the existence of a second chitinase in man, named acidic mammalian chitinase (AMCase) has opened the possibility that a deficiency in chitotriosidase might be partly compensated for by the presence of the latter enzyme (17). Recently, it has been demonstrated that AMCase, which is closely related to chitotriosidase, is involved in the pathology of an aeroallergen asthma model. Interference with AMCase activity in this model, either by addition of anti-sera or by addition of a chitinase-specific inhibitor, improves pathology (33). The inhibition of chitinase activity as a therapeutic approach for asthma has to be dealt with carefully as the risk exists that important anti-pathogenic activities of chitinases are eliminated as a consequence of total chitinase inhibition. Clinical data for instance show that chitotriosidase activity is raised in plasma of African children infected with acute Plasmodium falciparum malaria (34). Of note, in mice, chitotriosidase is not expressed by phagocytes but in the stratified squamous epithelium of the mucosal surface of the tongue, in the forestomach in the squamous epithelium layer and by Paneth cells in the crypts of Lieberkuhn (35).
In conclusion, the recent data on AMCase in asthma and the accumulating data on defence functions of chitotriosidase towards chitin-containing pathogens point towards an important role of the family of chitinase proteins in the human immune system and as interesting targets for therapeutic interventions.
Transmitting editor: J. Borst
We would like to acknowledge the stimulating discussions with late Gooday and P. L. Vieira for critical reading of the manuscript.
References
Hollak, C. E., van Weely, S., van Oers, M. H. and Aerts, J. M.
Renkema, G. H., Boot, R. G., Muijsers, A. O., Donker-Koopman, W. E. and Aerts, J. M.
Boot, R. G., Renkema, G. H., Strijland, A., van Zonneveld, A. J. and Aerts, J. M.
Renkema, G. H., Boot, R. G., Strijland, A. et al.
Boot, R. G., Renkema, G. H., Verhoek, M. et al.
Aguilera, B., Ghauharali-van der Vlugt, K., Helmond, M. T. et al.
Tharanathan, R. N. and Kittur, F. S.
Bradford, M.
Sarver, N., Gruss, P., Law, M. F., Khoury, G. and Howley, P. M.
Roberts, W. K. and Selitrennikoff, C. P.
van Pelt, L. J., Huisman, M. V., Weening, R. S., Von Dem Borne, A. E., Roos, D. and van Oers, R. H.
Nuijens, J. H., Abbink, J. J., Wachtfogel, Y. T. et al.
Kuijpers, T. W., Tool, A. T., van der Schoot, C. E. et al.
Jones, T. C.
Roilides, E., Lamaignere, C. G. and Farmaki, E.
Boot, R. G., Blommaart, E. F., Swart, E. et al.
Escott, G. M. and Adams, D. J.
Boussac, M. and Garin, J.
Bouzas, L., Carlos Guinarte, J. and Carlos Tutor, J.
Volck, B., Price, P. A., Johansen, J. S. et al.
Aerts, J. M., Hollak, C., Boot, R. and Groener, A.
Barone, R., Di Gregorio, F., Romeo, M. A., Schiliro, G. and Pavone, L.
Michelakakis, H., Dimitriou, E. and Labadaridis, I.
Renkema, G. H., Boot, R. G., Au, F. L. et al.
Boot, R. G., van Achterberg, T. A., van Aken, B. E. et al.
Hashimoto, S., Suzuki, T., Dong, H. Y., Yamazaki, N. and Matsushima, K.
Labadaridis, J., Dimitriou, E., Costalos, C. et al.
Malaguarnera, L., Simpore, J., Prodi, D. A. et al.
Choi, E. H., Zimmerman, P. A., Foster, C. B. et al.
Di Rosa, M., Musumeci, M., Scuto, A., Musumeci, S. and Malaguarnera, L.
Malaguarnera, L., Musumeci, M., Licata, F., Di Rosa, M., Messina, A. and Musumeci, S.
Zhu, Z., Zheng, T., Homer, R. J. et al.
Barone, R., Simpore, J., Malaguarnera, L., Pignatelli, S. and Musumeci, S.