-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea O. Rossetti, Sandrine Jeckelmann, Jan Novy, Patrick Roth, Michael Weller, Roger Stupp, Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study, Neuro-Oncology, Volume 16, Issue 4, April 2014, Pages 584–588, https://doi.org/10.1093/neuonc/not170
Close - Share Icon Share
Abstract
In patients with brain tumors, the choice of antiepileptic medication is guided by tolerability and pharmacokinetic interactions. This study investigated the effectiveness of levetiracetam (LEV) and pregabalin (PGB), 2 non-enzyme-inducing agents, in this setting.
In this pragmatic, randomized, unblinded phase II trial (NCT00629889), patients with primary brain tumors and epilepsy were titrated to a monotherapy of LEV or PGB. Efficacy and tolerability were assessed using structured questionnaires. The primary composite endpoint was the need to discontinue the study drug, add-on of a further antiepileptic treatment, or occurrence of at least 2 seizures with impaired consciousness during 1 year follow-up.
Over 40 months, 25 patients were randomized to LEV, and 27 to PGB. Most were middle-aged men, with a high-grade tumor and at least one generalized convulsion. Mean daily doses were 1125 mg (LEV) and 294 mg (PGB). Retention rates were 59% in the LEV group, and 41% in the PGB group. The composite endpoint was reached in 9 LEV and 12 PGB patients—need to discontinue: side effects, 6 LEV, 3 PGB; lack of efficacy, 1 and 2; impaired oral administration, 0 and 2; add-on of another agent: 1 LEV, 4 PGB; and seizures impairing consciousness: 1 in each. Seven LEV and 5 PGB subjects died of tumor progression.
This study shows that LEV and PGB represent valuable monotherapy options in this setting, with very good antiepileptic efficacy and an acceptable tolerability profile, and provides important data for the design of a phase III trial.
Seizures in patients with primary brain tumors represent a frequent problem, as at least one third of patients develop epilepsy in the course of disease.1,2 Subjects experiencing a first seizure should benefit from an antiepileptic drug (AED) prescription aimed at reducing further generalized convulsive events and improving quality of life.3 There is a wide consensus favoring the use of non-enzyme-inducing AEDs to avoid hepatic interactions potentially interfering with chemotherapy and other medications, such as steroids,4–8 but the level of evidence is still very limited, as recently pointed out.9 Furthermore, although all the newer AEDs were initially registered as add-on drugs in combination, recent experience suggests efficacy as single agents.
Among the newer AEDs, levetiracetam (LEV) and pregabalin (PGB) are considered among the most favorable compounds in terms of potential pharmacokinetic drug-drug interactions. LEV binds to a synaptic vesicle protein,10 lacks hepatic catabolism (it is excreted partly unchanged in the urine and partly undergoes extrahepatic hydrolysis),11 and is available in oral and intravenous formulations. It can be quickly titrated, displaying therapeutic efficacy within hours,12 and its bioavailability in the CSF seems longer than the plasmatic half-life.13 PGB modulates Q-type voltage-sensitive calcium channels,14 does not undergo any metabolic transformation (it has a complete renal excretion), and therefore does not have any impact on the hepatic cytochromic system.15,16 Despite being limited to oral formulations, it may also be titrated rapidly, exerting therapeutic efficacy within 48 h,17 and as opposed to gabapentin, which has an identical mechanism of action, PGB does not depend on a saturable intestinal resorption.18
This study was conducted to determine the safety and efficacy of AED monotherapy with LEV or PGB in patients with primary brain tumors and epilepsy and to collect prospective data on seizure control in brain tumor patients.
Materials and Methods
Design and Patients
This was a pragmatic open label phase II randomized trial conducted in 2 Swiss brain tumor centers. Subjects were included if aged >18 years, having a primary brain tumor (World Health Organization grades II–IV) provoking at least one previous seizure, therefore justifying introduction of AED treatment,19 and with a potential need of chemotherapy. All patients provided written informed consent. Exclusion criteria were status epilepticus on enrollment (defined as prolonged seizures >5 min needing emergent treatment20), need of an intravenous AED, requirement of a concomitant medication with another AED, a modified Rankin scale ≥4, known intolerance to the study drugs, preexistent psychosis, current suicidality, a life expectancy shorter than 4 weeks, or a current pregnancy (urine test was required on enrollment for women <50 y). Patients receiving other AEDs at the time of enrollment were eligible especially if they were inducing AEDs and if these could be discontinued within 2 weeks of enrollment; patients on phenobarbital were not included.
The study was approved by the ethics committees of the 2 participating Swiss university hospitals (Centre Hospitalier Universitaire Vaudois, University Hospital Zurich) and registered under Clinicaltrials.gov NCT00629889. Patients were randomized using a computerized predefined randomly drawn allocation series, and treatment was to be started at randomization.
Treatments
AEDs consisted of either LEV or PGB at increasing doses up to 2 × 1500 mg of LEV or 2 × 300 mg of PGB. The starting dose was: LEV 2 × 250 mg or PGB 2 × 75 mg, with increments (500 mg LEV, 150 mg PGB) in intervals of at least 24 h. Dose adaptation occurred at the discretion of the treating neurologist in accordance with standard practice. If needed, a transient prescription (<3 days) of benzodiazepine, but of no other AED, was allowed.
Follow-up and Procedures
The observation time within the trial was determined at 1 year. Fixed follow-up visits were scheduled at week 2 and after 2, 6, and 12 months of therapy. Baseline assessments included a full medical and neurological examination, electrocardiogram, electroencephalogram, brain MRI, a laboratory workup (including hematology, electrolytes, liver transaminases, creatinine), and seizure types and frequency. Potential adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 321 at baseline and during follow-up through a structured interview. The latter assessed the following: fatigue, somnolence (Epworth sleepiness scale), headache, dizziness, blurred vision, irritability, anxiety, psychosis, concentration problems, infections requiring antibiotics, skin rash, peripheral edema, subjective disturbance of cardiac rhythm, erectile dysfunction, and lowering of libido. Furthermore, during each visit patients were asked about current medication (compliance was categorized after subjective reports as optimal versus suboptimal if more than 1 dose/wk was omitted) and seizure type and frequency; they received a neurological examination and were weighed, arterial blood pressure and pulse were measured, and the modified Rankin scale was assessed. A laboratory workup was repeated on each visit (except 2 wk after enrollment).
Endpoints, Sample Size, and Statistics
The primary endpoint was survival free of the composite of the following endpoints, reflecting pragmatically the failure of an AED monotherapy: status epilepticus, 2 seizures with impaired consciousness, need of a second AED, need to discontinue the study drug (lack of efficacy or adverse reactions). Secondary endpoints were occurrence of any adverse event, occurrence of any component of the endpoint, and mortality. For adverse events, we considered any progression on the grading for any item during follow-up21; worsening of the Epworth sleepiness scale was categorized if an increase of ≥5 points was reported.
It was assumed that at the end of the 1-year follow-up, about 25% of patients would have experienced status epilepticus or 2 seizures with consciousness impairment, 15% would have experienced intolerable side effects, and 25% would need add-on treatment.3,22,23 Taking overlaps into account, the composite endpoint would have been reached by about 50% of study subjects. Targeting at least 10 subjects reaching the composite endpoint in each arm, and assuming that these would correspond to the lower limit of the 95% confidence interval (CI) using a binomial distribution, 30 patients would have been required in each arm.
Analysis was performed as intention to treat for patients receiving at least one study drug dose. The variables of interest were assessed using descriptive statistics (mean ± SD; median, range; or simple proportions, as needed), and Kaplan–Meier survival curves. Patients who died because of tumor progression or were lost to follow-up were censored. As the study was designed as a phase II trial, no comparison was undertaken between the 2 study drugs. Calculations were performed on Stata software v12.
Results
Between March 2008 and July 2011, 52 patients were enrolled in the 2 participating centers. Recruitment was stopped after reaching the composite endpoint in 21 subjects. Patient characteristics are depicted in Table 1. Most were middle-aged men, with a high-grade glioma, having presented with at least one generalized convulsion, having an abnormal neurological examination and electroencephalogram, and being very slightly clinically impaired. Slightly more than half of patients were on another AED (14 [56%] in the LEV arm, 13 [48%] in the PGB arm) before study entry: 10 on phenytoin, 6 on carbamazepine, 5 each on valproate and benzodiazepines, and 1 on lamotrigine. All patients had gliomas. Table 2 shows data regarding study medication and clinical evolution during the study. After 2 weeks, many patients were already on target drug dosage, which was further increased in the minority of them over the study; most reported good compliance with the study medication; about one-third experienced tumor progression.
Demographic and clinical characteristics of participating subjects on randomization
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Age, y, mean (±SD) | 54.5 (±12.7) | 52.7 (±10.9) |
| Female gender | 9 (36%) | 12 (44%) |
| Body mass index, kg/m2 | 26.1 (±6.6) | 23.6 (±6.0) |
| Brain tumor characteristics | ||
| High grade (WHO grades III–IV) | 17 (68%) | 20 (74%) |
| Recurrent tumor | 10 (40%) | 6 (22%) |
| Seizure types | ||
| Generalized convulsive | 14 (56%) | 15 (56%) |
| Focal | 11 (44%) | 10 (37%) |
| Generalized convulsive and focal | 0 (0%) | 2 (7%) |
| Modified Rankin scale, median (range) | 1 (0–3) | 1 (0–3) |
| Epworth sleepiness scale, median (range) | 4 (0–11) | 3 (0–13) |
| Abnormal neurological examination | 18 (72%) | 18 (67%) |
| Abnormal EEG | 20 (83%) | 21 (81%) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Age, y, mean (±SD) | 54.5 (±12.7) | 52.7 (±10.9) |
| Female gender | 9 (36%) | 12 (44%) |
| Body mass index, kg/m2 | 26.1 (±6.6) | 23.6 (±6.0) |
| Brain tumor characteristics | ||
| High grade (WHO grades III–IV) | 17 (68%) | 20 (74%) |
| Recurrent tumor | 10 (40%) | 6 (22%) |
| Seizure types | ||
| Generalized convulsive | 14 (56%) | 15 (56%) |
| Focal | 11 (44%) | 10 (37%) |
| Generalized convulsive and focal | 0 (0%) | 2 (7%) |
| Modified Rankin scale, median (range) | 1 (0–3) | 1 (0–3) |
| Epworth sleepiness scale, median (range) | 4 (0–11) | 3 (0–13) |
| Abnormal neurological examination | 18 (72%) | 18 (67%) |
| Abnormal EEG | 20 (83%) | 21 (81%) |
Abbreviations: WHO, World Health Organization; EEG, electroencephalogram.
Demographic and clinical characteristics of participating subjects on randomization
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Age, y, mean (±SD) | 54.5 (±12.7) | 52.7 (±10.9) |
| Female gender | 9 (36%) | 12 (44%) |
| Body mass index, kg/m2 | 26.1 (±6.6) | 23.6 (±6.0) |
| Brain tumor characteristics | ||
| High grade (WHO grades III–IV) | 17 (68%) | 20 (74%) |
| Recurrent tumor | 10 (40%) | 6 (22%) |
| Seizure types | ||
| Generalized convulsive | 14 (56%) | 15 (56%) |
| Focal | 11 (44%) | 10 (37%) |
| Generalized convulsive and focal | 0 (0%) | 2 (7%) |
| Modified Rankin scale, median (range) | 1 (0–3) | 1 (0–3) |
| Epworth sleepiness scale, median (range) | 4 (0–11) | 3 (0–13) |
| Abnormal neurological examination | 18 (72%) | 18 (67%) |
| Abnormal EEG | 20 (83%) | 21 (81%) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Age, y, mean (±SD) | 54.5 (±12.7) | 52.7 (±10.9) |
| Female gender | 9 (36%) | 12 (44%) |
| Body mass index, kg/m2 | 26.1 (±6.6) | 23.6 (±6.0) |
| Brain tumor characteristics | ||
| High grade (WHO grades III–IV) | 17 (68%) | 20 (74%) |
| Recurrent tumor | 10 (40%) | 6 (22%) |
| Seizure types | ||
| Generalized convulsive | 14 (56%) | 15 (56%) |
| Focal | 11 (44%) | 10 (37%) |
| Generalized convulsive and focal | 0 (0%) | 2 (7%) |
| Modified Rankin scale, median (range) | 1 (0–3) | 1 (0–3) |
| Epworth sleepiness scale, median (range) | 4 (0–11) | 3 (0–13) |
| Abnormal neurological examination | 18 (72%) | 18 (67%) |
| Abnormal EEG | 20 (83%) | 21 (81%) |
Abbreviations: WHO, World Health Organization; EEG, electroencephalogram.
Study medication and clinical evolution during study follow-up
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Daily drug dosage | ||
| At 2 wk, mg, mean (±SD) | 1000 (±505) | 239 (±82) |
| Maximum, mg, mean (±SD) | 1125 (±511) | 294 (±122) |
| Poor compliance | 2 (8%) | 7 (27%) |
| Brain tumor progression | 9 (38%) | 8 (31%) |
| Worst modified Rankin scale, median (range) | 1 (0–5) | 1 (0–5) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Daily drug dosage | ||
| At 2 wk, mg, mean (±SD) | 1000 (±505) | 239 (±82) |
| Maximum, mg, mean (±SD) | 1125 (±511) | 294 (±122) |
| Poor compliance | 2 (8%) | 7 (27%) |
| Brain tumor progression | 9 (38%) | 8 (31%) |
| Worst modified Rankin scale, median (range) | 1 (0–5) | 1 (0–5) |
Study medication and clinical evolution during study follow-up
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Daily drug dosage | ||
| At 2 wk, mg, mean (±SD) | 1000 (±505) | 239 (±82) |
| Maximum, mg, mean (±SD) | 1125 (±511) | 294 (±122) |
| Poor compliance | 2 (8%) | 7 (27%) |
| Brain tumor progression | 9 (38%) | 8 (31%) |
| Worst modified Rankin scale, median (range) | 1 (0–5) | 1 (0–5) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Daily drug dosage | ||
| At 2 wk, mg, mean (±SD) | 1000 (±505) | 239 (±82) |
| Maximum, mg, mean (±SD) | 1125 (±511) | 294 (±122) |
| Poor compliance | 2 (8%) | 7 (27%) |
| Brain tumor progression | 9 (38%) | 8 (31%) |
| Worst modified Rankin scale, median (range) | 1 (0–5) | 1 (0–5) |
After 1 year of follow-up, over one third of the patients failed therapy as defined per study endpoint: 9 in the PGB and 12 in the LEV group. Two subjects were censored, as they did not return for scheduled follow-up visits (1 early death due to tumor progression, 1 lost to follow-up). Table 3 summarizes the study endpoints. The composite endpoint was reached by more than one third of subjects: while no episode of status epilepticus and only 2 patients with occurrence of 2 seizures with impaired consciousness were recorded, in most cases the study drug was discontinued. This occurred in 7 patients in the LEV group (6 because of side effects: irritability, psychosis, dizziness, fatigue, headache, thrombopenia; 1 due to lack of efficacy) and 7 in the PGB group (3 for side effects: depression, sexual dysfunction, nausea; 2 because of impossibility to administer PGB orally due to tumor progression and worsening of clinical conditions; 2 because of lack of efficacy). Globally, 65% of patients on LEV and 75% on PGB remained seizure free until last follow-up.
Study endpoints
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Composite endpoint | 9 (36%) | 12 (44%) |
| Status epilepticus | 0 | 0 |
| 2 seizures with consciousness impairment | 1 (4%) | 1 (4%) |
| Need to interrupt study drug | 7 (28%) | 7 (26%) |
| Need to add on a second antiepileptic drug | 1 (4%) | 4 (15%) |
| Seizure free from enrollment until last follow-up | 17 (65%) | 18 (75%) |
| Lost to follow-up | 0 | 3 (11%) |
| Death | 7 (28%) | 5 (19%) |
| Survival in the study, days, median (range) | 286 (9–431) | 166 (0–410) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Composite endpoint | 9 (36%) | 12 (44%) |
| Status epilepticus | 0 | 0 |
| 2 seizures with consciousness impairment | 1 (4%) | 1 (4%) |
| Need to interrupt study drug | 7 (28%) | 7 (26%) |
| Need to add on a second antiepileptic drug | 1 (4%) | 4 (15%) |
| Seizure free from enrollment until last follow-up | 17 (65%) | 18 (75%) |
| Lost to follow-up | 0 | 3 (11%) |
| Death | 7 (28%) | 5 (19%) |
| Survival in the study, days, median (range) | 286 (9–431) | 166 (0–410) |
Study endpoints
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Composite endpoint | 9 (36%) | 12 (44%) |
| Status epilepticus | 0 | 0 |
| 2 seizures with consciousness impairment | 1 (4%) | 1 (4%) |
| Need to interrupt study drug | 7 (28%) | 7 (26%) |
| Need to add on a second antiepileptic drug | 1 (4%) | 4 (15%) |
| Seizure free from enrollment until last follow-up | 17 (65%) | 18 (75%) |
| Lost to follow-up | 0 | 3 (11%) |
| Death | 7 (28%) | 5 (19%) |
| Survival in the study, days, median (range) | 286 (9–431) | 166 (0–410) |
| . | Levetiracetam . | Pregabalin . |
|---|---|---|
| Randomized patients | 25 | 27 |
| Composite endpoint | 9 (36%) | 12 (44%) |
| Status epilepticus | 0 | 0 |
| 2 seizures with consciousness impairment | 1 (4%) | 1 (4%) |
| Need to interrupt study drug | 7 (28%) | 7 (26%) |
| Need to add on a second antiepileptic drug | 1 (4%) | 4 (15%) |
| Seizure free from enrollment until last follow-up | 17 (65%) | 18 (75%) |
| Lost to follow-up | 0 | 3 (11%) |
| Death | 7 (28%) | 5 (19%) |
| Survival in the study, days, median (range) | 286 (9–431) | 166 (0–410) |
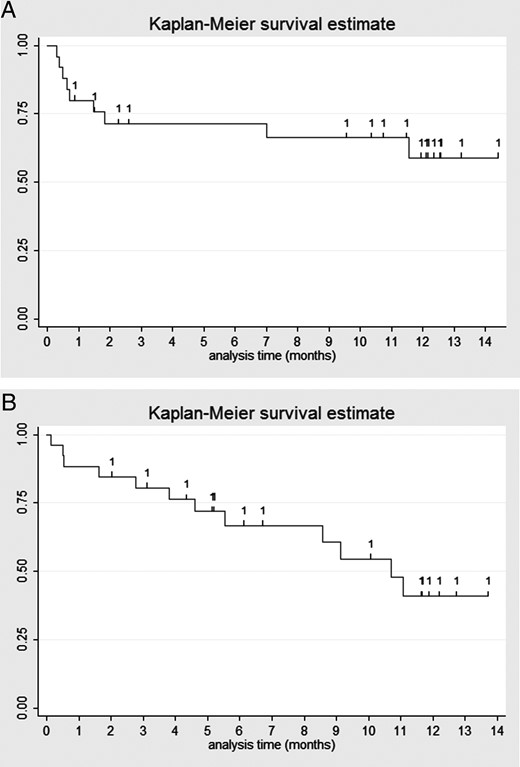
About one quarter of patients died during the study; no death was considered related to study drug. One patient taking LEV had a severe reduction in platelet counts that led to study discontinuation; this represented the only severe adverse event (and chemotherapy may have played the predominant role). The median retention of the allocated study drug was about 9.5 months in the LEV group and 5.5 months in the PGB group. Figure 1 illustrates the Kaplan–Meier curves for both medications. Nine patients (36%) in the LEV group and 7 (26%) in the PGB group were still taking their study medication after 1 year; considering censored patients, this corresponded to 59% in the LEV and 41% in the PGB group. Table 4 shows details regarding adverse events. Most frequently associated with LEV administration were somnolence, depression, and concentration problems; in the PGB group, dizziness, concentration problems, and depression. Conversely, erectile dysfunction, psychosis, skin rash, and weight increase were reported very rarely in either group. Overall, there were major hematological disturbances, probably related to concomitant chemotherapy.
New-onset adverse events occurring during follow-up
| . | Levetiracetam, % . | Pregabalin, % . |
|---|---|---|
| Leucocytes, grade worsening | 14.3 | 23.5 |
| Hemoglobin, grade worsening | 35.7 | 21.1 |
| Platelets, grade worsening | 30.8 | 22.2 |
| Epworth aggravation ≥5 points | 44.4 | 21.7 |
| Anxiety | 25.0 | 23.1 |
| Irritability | 12.5 | 15.4 |
| Depression | 37.5 | 26.9 |
| Psychosis | 4.2 | 0 |
| Concentration problems | 37.5 | 30.8 |
| Infection requiring antibiotics | 12.5 | 19.2 |
| Headache | 16.7 | 11.5 |
| Dizziness | 16.7 | 30.8 |
| Diplopia, blurred vision | 8.3 | 11.5 |
| Rash/desquamation | 0 | 7.7 |
| Weight increase >10% | 4.2 | 7.7 |
| Limb edema | 4.2 | 15.4 |
| Arrhythmia | 12.5 | 19.2 |
| Erectile dysfunction | 8.3 | 0 |
| Libido dysfunction | 20.8 | 23.1 |
| . | Levetiracetam, % . | Pregabalin, % . |
|---|---|---|
| Leucocytes, grade worsening | 14.3 | 23.5 |
| Hemoglobin, grade worsening | 35.7 | 21.1 |
| Platelets, grade worsening | 30.8 | 22.2 |
| Epworth aggravation ≥5 points | 44.4 | 21.7 |
| Anxiety | 25.0 | 23.1 |
| Irritability | 12.5 | 15.4 |
| Depression | 37.5 | 26.9 |
| Psychosis | 4.2 | 0 |
| Concentration problems | 37.5 | 30.8 |
| Infection requiring antibiotics | 12.5 | 19.2 |
| Headache | 16.7 | 11.5 |
| Dizziness | 16.7 | 30.8 |
| Diplopia, blurred vision | 8.3 | 11.5 |
| Rash/desquamation | 0 | 7.7 |
| Weight increase >10% | 4.2 | 7.7 |
| Limb edema | 4.2 | 15.4 |
| Arrhythmia | 12.5 | 19.2 |
| Erectile dysfunction | 8.3 | 0 |
| Libido dysfunction | 20.8 | 23.1 |
New-onset adverse events occurring during follow-up
| . | Levetiracetam, % . | Pregabalin, % . |
|---|---|---|
| Leucocytes, grade worsening | 14.3 | 23.5 |
| Hemoglobin, grade worsening | 35.7 | 21.1 |
| Platelets, grade worsening | 30.8 | 22.2 |
| Epworth aggravation ≥5 points | 44.4 | 21.7 |
| Anxiety | 25.0 | 23.1 |
| Irritability | 12.5 | 15.4 |
| Depression | 37.5 | 26.9 |
| Psychosis | 4.2 | 0 |
| Concentration problems | 37.5 | 30.8 |
| Infection requiring antibiotics | 12.5 | 19.2 |
| Headache | 16.7 | 11.5 |
| Dizziness | 16.7 | 30.8 |
| Diplopia, blurred vision | 8.3 | 11.5 |
| Rash/desquamation | 0 | 7.7 |
| Weight increase >10% | 4.2 | 7.7 |
| Limb edema | 4.2 | 15.4 |
| Arrhythmia | 12.5 | 19.2 |
| Erectile dysfunction | 8.3 | 0 |
| Libido dysfunction | 20.8 | 23.1 |
| . | Levetiracetam, % . | Pregabalin, % . |
|---|---|---|
| Leucocytes, grade worsening | 14.3 | 23.5 |
| Hemoglobin, grade worsening | 35.7 | 21.1 |
| Platelets, grade worsening | 30.8 | 22.2 |
| Epworth aggravation ≥5 points | 44.4 | 21.7 |
| Anxiety | 25.0 | 23.1 |
| Irritability | 12.5 | 15.4 |
| Depression | 37.5 | 26.9 |
| Psychosis | 4.2 | 0 |
| Concentration problems | 37.5 | 30.8 |
| Infection requiring antibiotics | 12.5 | 19.2 |
| Headache | 16.7 | 11.5 |
| Dizziness | 16.7 | 30.8 |
| Diplopia, blurred vision | 8.3 | 11.5 |
| Rash/desquamation | 0 | 7.7 |
| Weight increase >10% | 4.2 | 7.7 |
| Limb edema | 4.2 | 15.4 |
| Arrhythmia | 12.5 | 19.2 |
| Erectile dysfunction | 8.3 | 0 |
| Libido dysfunction | 20.8 | 23.1 |
(A and B) Kaplan–Meier curves showing retention of the study medication for LEV (1A) and PGB (1B); censored patients are marked.
Discussion
In this randomized phase II study, half of the enrolled patients remained on the allocated AED at 1 year; the primary reason of AED discontinuation was not lack of seizure control, but side effects. To the best of our knowledge, this is the first randomized controlled trial addressing seizure control of 2 non-enzyme-inducing agents used as monotherapy in brain tumor patients over 1 year.
Only 2 prospective studies assessing LEV monotherapy in similar settings have been conducted.22,24 Others tested LEV for 4 weeks in selected populations undergoing tumor resections25,26; the only randomized assessment is related to one trial focused on patients after craniotomy switched to LEV from phenytoin in a 2:1 ratio and followed for 6 months.27 Apart from our previous retrospective pilot analysis,23 no monotherapy study has been performed on PGB.
Reflecting its pragmatic approach, our study enrolled a population that was similar to other studies on the same topic in terms of demographics (mean age, 53 y; 60% men), brain tumor severity (71% high grade), and seizure type (generalizations in 56%).3,22–24 Twenty-three percent of patients died following brain tumor progression during the study; this is roughly similar to 34%,22 while no patient died at 4 weeks,26 and mortality was not reported in another study.24
The majority of the surviving patients remained seizure free beyond the last follow-up, underscoring that these monotherapies are well suited to treat epilepsy in brain tumor patients. Also, the marked efficacy of the study medications in preventing seizures with consciousness impairment confirms that AEDs prescribed in this setting are very successful in preventing convulsions.3 The target daily dose of LEV was almost reached within 2 weeks and remained relatively low (1125 mg). This is lower than in 2 Italian studies (mean: 1710 mg22; range: 1500–400024), probably reflecting different treatment policies and patients’ preferences. Conversely, the mean PGB daily dose (294 mg) is almost identical to a recent study (279 mg)28 using it, however, as an add-on.
In contrast to other studies, we assessed the AED retention rates at 1 year using Kaplan–Meier curves (59% for LEV and 41% for PGB); this analysis is more refined than crude percentages, since patients lost to follow-up or dying during the study (and thus “censored” for analysis) are still taken into account for the period during which they remained on the study medication. Therefore, a comparison with other studies may only be inferred indirectly: for LEV, patients still in the study were 100% of 17 patients (follow-up of 1 mo)26; 75% of 20 patients at 6 months27; 89% of 82 patients (follow-up 10–34 mo)24; and 52% of 29 patients (follow-up 1 y).28 While this broad variability, again, may reflect different practices and study designs, thresholds to discontinue a given study medication or add on a further AED may vary among different study scenarios. Interestingly, retention rates in our trial are similar to those reported from the same center for patients with epilepsy without tumors at 1 year: 70% for LEV29 and 50% for PGB.30
Although this study was not designed to compare retention rates of LEV and PGB, a qualitative analysis of the actuarial seizure control curves shows different patterns among these 2 agents. Patients on LEV tended to reach earlier (at about 50 days) a failure rate of 25%, while this was reached at about 130 days in the PGB group. However, subjects on PGB diminished somewhat constantly over time, while those on LEV tended to stabilize on the treatment after the first 2 months. While it is impossible to formally exclude that these patterns may reflect different attitudes of clinicians or participants toward the 2 agents, they might also inform upon the kinetics of the occurrence of side effects, with those following LEV appearing earlier. Furthermore, PGB was also discontinued due to impaired oral administration following tumor progression, which occurred late (after 9 and 11 mo).
The global prevalence of side effects in this study appears higher than in the 2 aforementioned studies focused on LEV: a transitory somnolence was reported as the only unwanted reaction in 4/82 patients (5%),24 while among 29 subjects, 3 had restlessness and 2 somnolence (total: 17%).22 These differences are likely due to the fact that our study used a structured interview and objective clinical data to investigate the side-effect profile in each patient, and therefore was probably more sensitive to adverse reactions, as opposed to assessments relying on self-reports. Moreover, it is virtually impossible, in this type of analysis, to sort out the events that are exclusively due to the AED and not to the underlying illness. For example, the high rate of hematological side effects in our data, clearly exceeding what is routinely observed in patients with epilepsy due to other causes, may reflect the profile of oncological medications, mostly temozolomide.31
LEV is well known to induce behavioral and psychiatric side effects in up to 15% of patients with epilepsy, in addition to some sedation.32 Accordingly, somnolence, depression, and concentration problems were the most frequently found items in our patients. Of note, LEV had been demonstrated to have fewer side effects than phenytoin in patients with brain tumors.27 PGB tolerability issues are related mainly to somnolence; conversely anxiolytic properties may represent a favorable effect.28,33 We observed mostly dizziness, concentration problems, and depression. While in our pilot study PGB was discontinued in 2/9 patients because of peripheral edema and erectile dysfunction,23 in the add-on study only 2/25 patients dropped out (dizziness, irritability).28
Our study has some limitations. Its sample size is fairly small, investigators and patients were not blinded to treatment, and some underlying heterogeneity of tumor and concomitant treatment was unavoidable. However, treatment allocation was random, and there was no obvious or detectable bias of patients or physician toward one of the assigned treatments. The real-life situation and pragmatic trial conduct may actually better reflect real-world clinical situations than do large trials on selected populations. Our patients' profiles were similar to other studies, underscoring its potential generalizability. The prospective design, with a structured and predefined assessment of side effects, represents a further strength. Last but not least, to date this is the largest series investigating PGB as monotherapy, and the only prospective study specifically addressing seizure control exclusively in tumor patients.
These results confirm that LEV and PGB represent valuable monotherapy options for the treatment of patients with epilepsy due to brain tumors, and provide robust data for the design of a large phase III trial among different AEDs in this clinical setting.
Funding
This study was supported by unrestricted grants from Pfizer and UCB Pharma.
Ackowledgments
The authors wish to thank D. Vulliémoz PhD, L. Pansier-Rossier PhD (Oncology, CHUV), and Drs M. Maeder-Ingvar, S. Jukopila, G. Di Virgilio, E. Accolla, J. Grujic, V. Stojanova, V. Alvarez, M. Spatola, C. Mössinger (Neurology, CHUV), as well as Drs K. Seystahl, H-G. Wirsching, and Verena Reichl, RN (Neurology, USZ) for their important help with data collection. Prof O. Michielin (Oncology, CHUV) kindly provided the randomization procedure.
Conflict of interest statement. None declared.
References
- anticonvulsants
- epilepsy
- seizures
- consciousness disturbance
- brain tumors
- consciousness related finding
- administration, oral
- phase 2 clinical trials
- phase 3 clinical trials
- follow-up
- middle-aged adult
- enzymes
- neoplasms
- levetiracetam
- generalized seizures
- primary brain tumors
- tumor progression
- pregabalin
- add-on code
- pharmacokinetic interaction