Article Text
Abstract
Objective: To assess the efficacy and safety of modafinil for the treatment of fatigue in multiple sclerosis (MS).
Methods: Patients aged 18–65 years with a diagnosis of MS, a stable disability level ≤6 on the Kurtzke extended disability status scale (EDSS), and a mean score >4 on the fatigue severity scale (FSS) were eligible for the 9 week, single blind, phase 2, two centre study. Exclusion criteria included a diagnosis of narcolepsy, sleep apnoea, or clinically significant major systemic disease and recent use of medications affecting fatigue. All patients, who remained blinded for the treatment regimen, received placebo during weeks 1–2, 200 mg/day modafinil during weeks 3–4, 400 mg/day modafinil during weeks 5–6, and placebo during weeks 7–9. Safety was evaluated by unblinded investigators. Efficacy was evaluated by self rating scales, using the FSS, the modified fatigue impact scale (MFIS), a visual analogue scale for fatigue (VAS-F), and the Epworth sleepiness scale (ESS). Adverse events were recorded.
Results: Seventy two patients (MS type: 74% relapsing-remitting; 7% primary progressive; 19% secondary progressive) received treatment. After treatment with 200 mg/day modafinil for 2 weeks, a significant improvement in fatigue versus placebo run in was demonstrated. Mean scores after treatment with 200 mg/day modafinil were: FSS, 4.7 versus 5.5 for placebo (p<0.001); MFIS, 37.7 versus 44.7 (p<0.001); and VAS-F, 5.4 versus 4.5 (p=0.003). Fatigue scores for 400 mg/day modafinil were not significantly improved versus placebo run in. Mean ESS scores were significantly improved (p<0.001) with 200 mg/day modafinil (7.2) and 400 mg/day (7.0) versus the score at baseline (9.5). Serious adverse events were not found at either dose. The most common adverse events were headache, nausea, and aesthenia. Sixty five patients (90%) completed the study.
Conclusions: These data suggest that 200 mg/day modafinil significantly improves fatigue and is well tolerated in patients with MS.
- multiple sclerosis
- fatigue
- modafinil
- MS, multiple sclerosis
- EDSS, Kurtzke extended disability status scale
- FSS, fatigue severity scale
- MFIS, modified fatigue impact scale
- VAS-F, a visual analogue scale for fatigue
- ESS, Epworth sleepiness scale
- SSRIs, selective serotonin reuptake inhibitors
- EDS, excessive daytime sleepiness
- POMS, profile of mood states
Statistics from Altmetric.com
- MS, multiple sclerosis
- EDSS, Kurtzke extended disability status scale
- FSS, fatigue severity scale
- MFIS, modified fatigue impact scale
- VAS-F, a visual analogue scale for fatigue
- ESS, Epworth sleepiness scale
- SSRIs, selective serotonin reuptake inhibitors
- EDS, excessive daytime sleepiness
- POMS, profile of mood states
Fatigue, defined as a subjective lack of physical or mental energy to carry out usual and desired activities as perceived by the patient or caregiver,1 is common and one of the most disabling symptoms of multiple sclerosis (MS). Fatigue affects 75%-90% of patients with MS,2–,5 with as many as 46%-66% experiencing fatigue on a daily basis.4,6 Unfortunately, despite its high prevalence, chronic nature, and association with disability, there is currently no accepted treatment for MS related fatigue. Various pharmacological agents have been used for its treatment, including amantadine, pemoline, aminopyridines, and selective serotonin reuptake inhibitors (SSRIs). Four short term studies indicated that fatigue was reduced with amantadine treatment in 20%-40% of patients with MS who had mild to moderate disability.5,7–,9 Studies have suggested that high dosages of pemoline (75 mg/day) are needed to achieve any improvement in MS related fatigue,7,10 but even with a high dosage the improvement was not significant when compared with placebo treatment. Although sometimes used in the treatment of MS related fatigue, no controlled clinical studies of the efficacy and safety of aminopyridines or SSRIs have been published. Therefore, the use of these agents for the treatment of MS related fatigue is not recommended.1
Modafinil, a novel wake promoting agent, is effective and well tolerated for the treatment of excessive daytime sleepiness (EDS) in patients with narcolepsy.11–,13 After 9 weeks of double blind treatment with 200 mg/day and 400 mg/day modafinil in two pivotal double blind studies of modafinil for the treatment of EDS in patients with narcolepsy, the mean SF-36 vitality scores significantly improved by 11 points and 13 points over the baseline scores.14 The improvements in vitality found after treatment with modafinil were significant (p<0.05 compared with placebo) and were maintained during 40 weeks of subsequent open label treatment with modafinil.14 In a 6 week open label trial of 151 patients with narcolepsy who had been unsatisfactorily treated for EDS with CNS stimulants, treatment with modafinil significantly improved wakefulness and also significantly improved fatigue as assessed using both the SF-36 and the profile of mood states (POMS) questionnaire.15 Therefore, it was of interest to investigate the effect of modafinil on the fatigue experienced by patients with MS. We report here, the results of our study that examined the efficacy and safety of modafinil for the treatment of fatigue in patients with MS.
METHODS
Study design
A 9 week, single blind, pilot study was conducted at two sites. A single blinded design was preferred as modafinil had not been previously studied in patients with MS, and the safety of the use of this agent in MS was not known. The patients, but not the investigators, were blinded to the treatment to permit the detection of any untoward and unanticipated side effects. Because the optimum dose of modafinil was unknown, a forced titration design was chosen. The study protocol was approved by the institutional review board at each site. All participants were told that they would receive active drug during the trial but would be blinded to the treatment sequence. Each patient provided written informed consent before enrolment. After a baseline visit (day 0), all patients who were enrolled in the study received matching placebo (four tablets) during the first 2 weeks (phase 1), 200 mg/day modafinil (two 100 mg tablets) plus two placebo tablets during weeks 3 and 4 (phase 2), 400 mg/day modafinil (four 100 mg tablets) during weeks 5 and 6 (phase 3), and four matching placebo tablets during weeks 7–9 (phase 4). Study medication was provided in blister packs containing a daily dose of four tablets (100 mg modafinil and/or matching placebo) that were identical in appearance. Study medication was taken once daily in the morning.
Sample size estimation
Sample size estimates were performed to show group mean differences between placebo and treatment groups of one point on the fatigue severity scale (FSS) using a two tailed test. It was determined that a sample size of 72 (36 subjects/site) would be required. This would achieve a power of about 80% in detecting a treatment difference of one point on the FSS between modafinil and placebo run in at a significance level of 5% and an anticipated drop out rate of 20%.
Patients
Men and women aged 18–65 years with a diagnosis of MS, a stable disability level of 6 or less on the Kurtzke extended disability status scale (EDSS),16 and a mean score of 4 or more on the FSS17 were eligible for enrolment in the study. Exclusion criteria included a diagnosis of narcolepsy, sleep apnoea, hypothyroidism not adequately controlled with medication, blood pressure greater than 150/100 mm Hg, severe depression (defined as a score of >35 on the Center for Epidemiological Studies depression scale), or clinically significant major systemic disease. Patients were also excluded if they had a history of alcohol, narcotic, or other drug misuse, consumed excessive amounts of beverages or foods containing caffeine, or were using medications affecting fatigue. In addition, the following concomitant medications affecting fatigue were not allowed during the study: amantadine, methylphenidate, antipsychotic agents, amphetamines, pemoline, phenobarbital, tizanidine, monoamine oxidase inhibitors, anticoagulant drugs, benzodiazepines, barbiturates, tricyclic antidepressant drugs, SSRIs, and antihistamines other than astemizole, fexfenadine, or loratadine. Patients enrolled in the study were permitted to continue using disease modifying therapies for MS (for example, interferon-β, glatiramer acetate).
Outcome variables
Efficacy was evaluated at the baseline visit and after each treatment phase using the following self administered measures of fatigue: (1) the 9 item FSS17 (score range 1–7, with lower scores indicating less fatigue), (2) the 21 item modified fatigue impact scale3,18 (MFIS; score range 0–84, with lower scores indicating less fatigue), and (3) a visual analogue scale for fatigue (VAS-F; score range 0–10, with higher scores indicating less fatigue). The FSS, the primary efficacy variable in the study, has been shown to have a high degree of internal consistency, validity, and sensitivity to changes in clinical condition.17 The MFIS, a shortened version of the FIS,3 has been recommended by the fatigue guidelines development panel of the MS Council for Clinical Practice Guidelines as the main outcome measure for assessing MS related fatigue,1 but has not been validated previously in a clinical trial. The VAS-F, which was used in the Canadian MS Research Group trial,13 differs from the FSS and MFIS scales in that it provides a global impression of fatigue and does not focus on the impact of fatigue on specific, selected activities.
In addition to the three fatigue scales, patients completed the 8 item Epworth sleepiness scale19 (ESS, score range 0–24, with lower scores indicating less sleepiness) at the baseline visit (day 0) and after each modafinil treatment phase (week 4 and week 6). Adverse events, together with their severity and perceived relation to study medication, were recorded throughout the study. Serious adverse events (for example, those requiring admission to hospital or that resulted in a persistent or significant disability or incapacity) were also recorded.
Statistical analysis
Analysis of efficacy for each treatment phase was performed using intention to treat data obtained from patients who received at least one dose of study medication and had at least one assessment of efficacy during the particular treatment phase. Data were analyzed using the statistical analysis system (SAS) software package for windows (version 6.12). The overall mean fatigue score (FSS) or the mean total fatigue score (MFIS and VAS-F) was calculated for each treatment phase. For the MFIS, the mean scores for the physical (nine questions), cognitive (10 questions), and psychosocial (two questions) subscales were also calculated for each treatment phase. Statistical comparisons were made between mean scores for placebo run in (phase 1) and mean scores for 200 mg/day modafinil (phase 2) or 400 mg/day (phase 3) using an analysis of variance (ANOVA) model applicable to repeated measures data. This ANOVA model included terms for age, treatment effect, study centre effect, and subject within centre effect. There were no significant treatment by centre interactions for any of the four efficacy measures. For each fatigue scale, a post hoc subgroup analysis of the data were also performed based on the ESS score (>8 or ≤8) at the baseline visit. Most normal volunteers have ESS scores that are equal to or less than 8.19 Statistical comparisons for the fatigue scale data from the subgroup analysis were performed as described above. For the ESS, the overall mean score was calculated and statistical comparisons were made between the mean score for each modafinil dosage and the mean score at the baseline visit using ANOVA as described above. All statistical tests were two sided and conducted at the 5% significance level.
RESULTS
Demographics
A total of 72 patients with MS (18 men and 54 women; 36/site) were included in the study (table 1⇓). Most of the patients (74%) had relapsing-remitting MS. At baseline, the mean duration of MS was 6.3 years and the mean EDSS score was 3.3. Fifty five patients (76%) were receiving maintenance therapy with interferon-β or glatiramer acetate. Compliance, as determined by tablet counts, was uniformly high across all four treatment phases of the trial (mean range 87% to 89%).
Baseline characteristics
Efficacy assessments
After receiving 200 mg/day modafinil for 2 weeks, patients showed a significant improvement in fatigue compared with placebo run in treatment. The overall mean score of the nine item FSS showed improvement with significantly lower scores after treatment with 200 mg/day modafinil (4.7) than after treatment with placebo (5.5, p<0.001) (fig 1⇓). The overall mean FSS score after treatment with 400 mg/day modafinil increased to 5.3, which was marginally lower than but not significantly different from the mean score after treatment with placebo in phase 1. After 3 weeks of placebo treatment during phase 4 (wash out), the mean FSS score was also 5.3.
Mean scores (SEM) of the fatigue severity scale (FSS) at the end of each treatment phase. The dashed line represents the mean score at the baseline visit. * A significant improvement in fatigue versus the placebo run in phase (p<0.001).
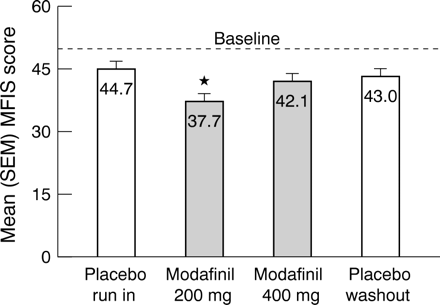
For the 21 item MFIS, the mean total score after treatment with 200 mg/day modafinil was 37.7 compared with 44.7 after treatment with placebo (p<0.001) (fig 2⇓). The mean scores for the physical, cognitive, and psychosocial subscales of the MFIS were also significantly improved after treatment with 200 mg/day modafinil compared with those after the placebo run in phase (all p<0.001). The mean total MFIS score (fig 2⇓) and the mean scores of the three MFIS subscales seen after treatment with 400 mg/day modafinil were not different from those seen after the placebo run in phase. After 3 weeks of placebo treatment during phase 4 (wash out), the mean total MFIS score was roughly equal to the mean total score at the end of the placebo run in phase.
Mean scores (SEM) of the modified fatigue impact scale (MFIS) at the end of each treatment phase. The dashed line represents the mean score at the baseline visit. *A significant improvement in fatigue versus the placebo run in phase (p<0.001).
The results of the VAS-F also showed a significant improvement in MS related fatigue after treatment with 200 mg/day modafinil (fig 3⇓). The mean score after treatment with 200 mg/day modafinil was 5.4 compared with 4.5 after the placebo run in phase (p=0.003). The mean score after treatment with 400 mg/day modafinil (4.7) and after 3 weeks of placebo during phase 4 (wash out) did not differ significantly from the mean score after the placebo run in phase.
Mean scores (SEM) of the visual analogue scale for fatigue (VAS-F) at the end of each treatment phase. The dashed line represents the mean score at the baseline visit. *A significant improvement in fatigue versus the placebo run in phase (p=0.003).
Daytime sleepiness, as measured with the eight item ESS, was significantly improved with both the 200 mg/day and the 400 mg/day doses of modafinil (fig 4⇓). The total mean scores were 7.2 and 7.0 after treatment with 200 mg/day and 400 mg/day modafinil, respectively, compared with the baseline mean score of 9.5 (all p<0.001). The results of the subanalysis based on the patients' ESS score at the baseline visit (≤8 or >8) indicated a tendency for patients with baseline ESS scores greater than 8 (greater sleepiness) to have poorer FSS and MFIS scores at the baseline visit. However, the improvements in fatigue from the placebo run in phase that were found after treatment with 200 mg/day modafinil, as measured with the FSS (4.3 v 5.2 for ESS ≤8; 4.9 v 5.7 for ESS >8) and MFIS (32.8 v 40.2 for ESS ≤8; 40.6 v 47.5 for ESS >8), were significant (all p<0.05) and of the same magnitude for the two subgroups. Regression analyses of ESS scores versus fatigue scores and change in ESS scores versus change in fatigue scores did not disclose an association between sleepiness and fatigue.
Mean scores (SEM) of the Epworth sleepiness scale (ESS) at baseline and at the end of each modafinil treatment phase. *A significant improvement in sleepiness versus the value at baseline (p<0.001).
Safety assessments
Sixty five of the 72 patients (90%) who were enrolled completed all four treatment phases of the study. The most frequent adverse events during the 200 mg/day modafinil treatment phase were headache, nausea, and anxiety (table 2⇓). All of the adverse events experienced during treatment with 200 mg/day modafinil were mild (63%) or moderate (37%) in nature. During treatment with 400 mg/day modafinil, the most frequent adverse events were asthenia (described as “worsened fatigue”; 14% v 3% with 200 mg/day modafinil and 8% for placebo run in), headache, nausea, and nervousness. The adverse events experienced during treatment with 400 mg/day modafinil were predominantly mild (58%) or moderate (39%) in nature. No serious adverse events were reported during any treatment phase.
Most common adverse events and reasons for discontinuation of treatment according
During the trial, five patients reported a worsening of MS symptoms. Three patients reported worsening while receiving 200 mg/day modafinil (1 mild and 2 moderate). These adverse events were considered possibly related to treatment, did not require initiation of treatment with steroids, and resolved without adjustment of modafinil dosage. One patient reported severe and continuing exacerbation of symptoms during the placebo run in phase, discontinued the study before receiving modafinil, and received steroid treatment for 5 days. Another patient reported a worsening of symptoms during the placebo wash out period, which was considered to be unrelated to study medication.
Six patients discontinued treatment because of adverse events (one during placebo run in, four during 400 mg/day modafinil, and one during placebo wash out phase) and one patient was lost to follow up during treatment with 200 mg/day modafinil. The four patients who discontinued treatment with 400 mg/day modafinil reported headache (n=2), dry mouth (n=2), anxiety (n=2), nausea (n=1), dizziness (n=1), palpitation (n=1), insomnia (n=1); depersonalisation (n=1), agitation (n=1), and sexual function abnormality (n=1).
DISCUSSION
In the present study, treatment with 200 mg/day modafinil for 2 weeks resulted in a significant improvement in fatigue scores for all three fatigue scales when compared with scores obtained after the placebo run in phase. It is important to note that whereas other agents used to treat MS fatigue, such as amantadine and pemoline, have been evaluated in previous clinical trials7 with the same fatigue scales, neither of those therapies have shown the same magnitude of improvement as seen in this study. In fact, neither treatment resulted in statistically significant effects when compared with treatment with placebo.
Although the 200 mg dose was effective, the mean fatigue scores on each scale were not significantly different from the mean fatigue scores after the placebo run in phase when modafinil was tested at 400 mg/day. Why the significant improvements in MS related fatigue with the 200 mg/day dosage of modafinil were not maintained with the 400 mg/day dosage is unclear. Several explanations were considered. These include development of tolerance or tachyphylaxis or adverse effects at the higher doses masking any possible benefits on fatigue. The possibility that the effect of modafinil treatment on fatigue may be self limiting cannot be ruled out by the current investigation. However, results of treatment of fatigue in patients with narcolepsy do not support this position. Open label, flexible dose studies of modafinil in patients with narcolepsy indicate that the effect of modafinil on fatigue15 and vitality (energy level/fatigue)14,20 are improved within the first few weeks of treatment. In an open label trial of modafinil in patients with narcolepsy, fatigue improvement was maintained for 6 weeks15 and improvements in vitality were maintained for periods of up to 88 weeks with 200 mg/day and 400 mg/day modafinil in other open label trials.14,20 Additionally, tolerance to the beneficial effects of modafinil on fatigue has not been reported by our patients who continue to receive modafinil for periods in excess of a year. Although the overall incidence of adverse events was similar during the 200 mg/day and 400 mg/day modafinil treatment phases, it is possible that adverse effects at the higher dose, including a worsening of fatigue, masked the benefits of treatment with modafinil. Finally it should be noted that some patients preferred the 400 mg dose to the 200 mg dose regarding the improvements in fatigue found during the study. The issue regarding the ideal dose of this agent is best resolved by titrating to the dose that provides the most benefit with the fewest intolerable or unacceptable adverse effects. It is quite likely that in some patients the effective dose for improvement of fatigue may indeed be 400 mg/day.
Our data also suggest that the dose-response of modafinil in the treatment of fatigue may be different in MS than the doses for treatment of excessive daytime sleepiness associated with narcolepsy. A possible “inverted U effect” similar to the data reported here has been demonstrated in animal studies, where modafinil at doses of 30 mg/kg improved cognitive function in aged rats but at higher doses (60 mg/kg), the magnitude of improvement was smaller.21 At the same time the “inverted U” effect was not found in these animals when improvements in sleepiness was determined using the same dosing conditions. Similarly, decreased feeding and weight loss have been demonstrated in rats receiving 20 or 40 mg/kg modafinil, but not in rats receiving 10 or 80 mg/kg.22 Collectively, these findings could suggest that modafinil effects that mediate wakefulness differ from those that mediate cognition or perhaps fatigue.
Daytime sleepiness, as assessed with the ESS, was significantly decreased after treatment with both 200 mg/day and 400 mg/day modafinil compared with placebo. The mean ESS score before treatment in the patients with MS related fatigue was 9.5 (SEM 4.4), which is markedly higher than the mean score for normal people (4.5 (SD 3.3).23
Sleep disorders and sleep disruption are known to occur in patients with MS and have been proposed as factors that contribute to MS related fatigue. However, because many patients with MS who report fatigue do not have a sleep disorder, other causes of fatigue must also be involved. Confounding this issue is the finding that many people have a difficult time distinguishing between fatigue and sleepiness. In a study of 309 patients with MS who experienced symptomatic fatigue,4 90% described their fatigue as tiredness or a need to rest, but 43% reported sleepiness as a contributing factor. Interestingly, studies have suggested that there is no relation between disrupted sleep and severity of daytime fatigue in patients with MS.6,7 In the present study, patients with higher ESS scores at baseline (> 8; greater sleepiness) had fatigue scores at baseline that were indicative of greater fatigue. However, the improvement in fatigue with modafinil treatment did not correlate well with ESS scores at baseline. Finally, sleepiness improved significantly with 400 mg/day modafinil, whereas fatigue did not. Thus, the relation between fatigue and sleepiness in patients with MS remains unclear.
Assessment of the safety profile of modafinil in patients with MS was of primary consideration in the design of this study, and the results of the trial show that treatment with modafinil was well tolerated. The most common adverse events during treatment with modafinil were headache, nausea, and asthenia. The adverse events associated with modafinil treatment were primarily mild and transient in nature. The 400 mg dose was associated with increased reports of asthenia. Aesthenia occurred more often with 400 mg/day doses of modafinil (10 reports) than with 200 mg/day doses of modafinil (two reports). Four patients stopped treatment with modafinil because of adverse events; all were receiving 400 mg/day doses of modafinil. Thus, our overall impression of the safety profile of modafinil in this study is that patients may have been less tolerant of the adverse events experienced on 400 mg/day modafinil compared with the adverse events experienced while receiving 200 mg/day, or that the adverse events reported while receiving 400 mg/day may have been more severe in some patients than was reflected in the recorded severity ratings. Pending appropriate clinical trials evaluating dose and duration of response to fatigue, doses of modafinil should be individualised based on clinical response.
This is the first study to evaluate the safety and efficacy of modafinil in MS related fatigue. A randomised double blind design was not considered suitable as the safety of modafinil treatment in conjunction with interferons and glatiramer was unknown, and the ideal dose in patients with MS was yet to be defined. The design used in this study assured that the patients would not be at risk because of unexpected or unanticipated adverse events. Modafinil treatment was well tolerated by the patients in this study and 76% of these patients were taking interferons or glatiramer acetate. Furthermore, there were no adverse events that seemed to be related to drug-drug interactions, suggesting that the combination of modafinil with interferon-β or glatiramer acetate therapy is well tolerated. These safety findings, together with the known safety profile of modafinil, suggest that future studies employing a crossover or randomised parallel group design would not constitute a safety risk.
This study provides preliminary results indicating that modafinil may be a well tolerated and effective treatment for fatigue in patients with MS. Modafinil may represent an important addition to the pharmacological treatment options available for the management of MS related fatigue. Additional trials to assess the long term safety and efficacy of modafinil for the treatment of fatigue are warranted.
Acknowledgments
This study was funded by Cephalon, and supported by Ohio State University General Clinical Research Center (NIH grant M01-RR00034).