Article Text
Abstract
Objective Is health-related quality of life 12 months after randomisation in participants with functional neurological symptoms better after discussion of the diagnosis by trained neurologists who schedule at least two follow-up visits (intervention group) than after the same discussion of the diagnosis by these neurologists and immediate referral to the general practitioner (control group)?
Methods A single-centre randomised controlled trial at one academic outpatient department of neurology. Participants were randomised 1:1, stratified for type of functional symptoms. The study sample consisted of 100 participants in the intervention group, and 95 participants in the control group. Primary outcome was the mean change 36-Item Short Form Health Survery (SF-36) scores from baseline to 12 months.
Results Participants in both treatment groups showed improvements on most SF-36 subscales and secondary outcomes measures but without significant between-group differences in mean change scores. Neither was there a difference between the treatment arms with regard to the number of participants who reported their symptoms at 12 months to have greatly improved compared with baseline: 29 participants (29/98=29.6%; two missing values) in the intervention group versus 31 participants (31/95=32.6%) in the control group (95% CI of the difference between proportions: from −16.1% to 10%).
Conclusion This study showed that after a neurologist has established the diagnosis and briefly explained and thereafter has sent the patient to a neurologist with a special training who scheduled half an hour to discuss the diagnosis, more sessions by this neurologist do not improve outcome.
Clinical trial registration number : NTR 2570.
- functional neurological symptoms
- conversion disorder
- psychogenic symptoms
- randomised controlled trial
Statistics from Altmetric.com
Introduction
After the diagnosis functional neurological symptoms (FNSs)1 has been established, in the Netherlands these patients are referred for treatment to general practitioners (GPs) or to psychiatrists or psychologists.2 At our centre, neurologists who had established the diagnosis FNS referred these patients for explanation of the diagnosis to neurologists who had a special training to explain the diagnosis FNS. These neurologists felt this explanation has to be repeated several times. The aim of this study was therefore to investigate whether health-related quality of life 12 months after randomisation is better after explanation of the diagnosis by neurologists with a special training and several follow-up visits than after explanation by these neurologists and immediate referral to the GP. In this trial to all patients, the diagnosis was explained according to a guideline, and web information on FNS was used.3
Methods
Participants and setting
We performed a single-centre randomised controlled trial at the outpatient department of neurology of the Academic Medical Center (AMC), Amsterdam, The Netherlands, in participants with FNS. Participants had been referred to the Department of Neurology by GPs. All persons in whom the diagnosis FNS was considered, had a thorough neurological assessment. Ancillary investigations to exclude other neurological explanations for the symptoms were left at the discretion of the neurologists. Subjects were eligible to participate if one of the following clusters of symptoms were present: (1) pain: tension-type headache (headache without alarming symptoms and not consistent with one of the headache syndromes such as migraine, analgesic abuse and cluster headache) and at least one other FNSs; back or neck pain (pain not caused by spinal pathology such as fractures, spondylitis and metastases; myelopathy; radiculopathy; plexopathy or neuropathy) and at least one other functional symptom; (2) ‘pseudo’neurological symptoms: functional movement disorders (movement disorders not consistent with known ‘organic’ movement disorders); motor impairment other than in movement disorders (motor impairment that cannot be explained by central or peripheral nervous system disorders) and/or sensory impairment (loss of sensory perception that can neither be explained by central nor by peripheral nervous system disorders); dissociative attacks or psychogenic non-epileptic seizures (seizures without evidence for epilepsy on electroencephalograms) and (3) ‘positive’ sensory symptoms: hyper sensory perception that can neither be explained by central nor by peripheral nervous system disorders.
We excluded subjects <18 years; if the duration of the functional symptoms since the first consultation at the office of the GP was >1 year; patients known to have psychiatric disorders other than somatoform, depressive or anxiety disorders; patients with a primary diagnosis of a severe mood, generalised anxiety or psychotic disorder requiring psychiatric treatment; patients treated with psychotherapy; patients known to simulate the symptoms; those who are in dispute about financial or social benefit; patients suffering from a major somatic disease; and persons with insufficient understanding of the Dutch language.
Patient involvement
Patients/carers/lay people were not involved in the design, the recruitment to or conduct of the study. The study participants were informed about the results by letter.
Blinding and randomisation
Participants were included according to the postponed informed consent procedure.4 5 After having established the diagnosis FNS and having checked the inclusion and exclusion criteria, neurologists briefly discussed the diagnosis and informed the participants about referral to neurologists who would check the diagnosis and who would discuss treatment options. These neurologists, to whom participants were referred, had had training in explaining the diagnosis and were interested in FNS. They extensively discussed the diagnosis FNS for which half an hour was scheduled. Before discussing management options, the person was asked to participate in a study in which persons with a similar diagnosis fill out self-reported health questionnaires on entry and after 3, 6 and 12 months. The participant was also informed that there was an additional question of which information would be given at the end of the study (postponed informed consent). If he or she agreed to participate, the neurologist left the room temporarily to collect the informed consent form and to randomise the person (initial management by the neurologist or immediate referral to the GP). The randomisation procedure was web based (using a validated TENALEA Clinical Trial Data Management System). Randomisation in a 1:1 ratio was stratified for type of functional symptoms (pain, ‘pseudo’neurological symptoms or ‘positive’ sensory symptoms) with permuted blocks within the strata.
Interventions
Standard management
The neurologist with special training to explain FNS discussed the diagnosis FNS with all enrolled participants following a guideline.3 If the participants wished to have more information, they were referred to a website, www.neurosymptoms.org, which after inclusion of the first 20 participants, a Dutch version became available. For explanation of the diagnosis, a baseline visit of 30 min was planned. After this information, the participants were randomised to initial management by the neurologist or to immediate management by the GP.
Initial management by the neurologist
The neurologist with special training to explain FNS planned at least two follow-up outpatient visits of 30 min at 6-week intervals. If the participant wished more visits, this was possible at 6-week intervals. At these follow-up visits, the neurologist again asked what the thoughts of the patients were on the diagnosis and tried to correct these if necessary. In addition, patients were encouraged to gradually increase their activities of daily life. If the progress with the daily activities was considered too slow, the patients were referred to a physiotherapist. Referral for psychotherapy was left to the discretion of the neurologist. The neurological care at baseline and follow-up visits were given by four neurologists; about 90% of the participants was treated by one neurologist (MV).
Immediate management by the GP
The GP was informed by the neurologist about the diagnosis and the information that was given to the participant and that the participant was referred back for further management. Referral to other professionals was left to the discretion of the GP. The GP was also informed that the person participated in a study in which patients with a similar diagnosis filled out postal health questionnaires with queries on the condition of the patients and that these questionnaires were sent to the patients after 3, 6 and 12 months. The GPs were not informed about the randomisation.
Data collection
At baseline, a set of self-reported health questionnaires was filled out by the participants during their visit at the outpatient clinic. After 3, 6 and 12 months, the same set of health questionnaires was sent to the participants by post. The research nurse checked the returned questionnaires for completeness. If not complete, the research nurse contacted the participant by telephone for further information. If the participant had not returned the questionnaire, the research nurse also contacted the participant by telephone and stressed the importance of these questionnaires for the study. In case the questionnaire had not been returned after 3 months, the participant was contacted again 3 months later. If the questionnaire had not been returned after 6 months, the participant was asked to at least fill out the 12-month questionnaire and the research nurse offered support in filling out the final forms. If necessary, the neurologist asked the GP to motivate the patient to fill out the 12-month questionnaires.
Outcomes
Primary outcome
The primary outcome was the participant’s health-related quality of life 12 months after randomisation. Health-related quality of life (HRQL) was assessed using the 36-item Short Form Health Survey (SF-36).6 Two dimension scores can be derived from the subscale scores: the physical component summary and the mental component summary.7 Further details of the SF-36 are presented in online supplementary appendix 1.
Secondary outcomes
Secondary outcomes at 12-month follow-up were severity of pain, assessed on a 100 mm horizontal Visual Analogue Scale (VAS), the Pain Catastrophizing Scale (PCS),8 the somatisation subscale of the Symptom Check List (SCL)-90,9 the Hospital Anxiety and Depression Scale (HADS),10 the AMC Linear Disability Scale (ALDS)11 and general well-being measured on a 100 mm horizontal VAS (see online supplementary appendix 1). Participants scored their perceived change of symptoms during the past 12 months into the following five categories: greatly improved, somewhat improved, remained the same, somewhat deteriorated and greatly deteriorated. In addition, the use of physical or psychotherapy was recorded.
Sample size calculation
For the primary HRQL outcome, we used Cohen’s d effect size (12-month difference between the mean SF-36 scores of the intervention group and the control group divided by the pooled SD) as benchmark for the relative magnitude of score differences between both strategies. Although an effect size of d=0.50 can be defined as moderate,12 such a quality-of-life score may be clinically important. With a sample size of 200 participants (100 per treatment arm), we had 90% power to detect an effect size of d=0.50, using a two-group Student’s t-test with a 0.05 two-sided significance level.
Statistical analysis
Data were analysed according to the intention-to-treat principle. Baseline characteristics and outcome parameters were summarised using descriptive statistics. As both the 3-month and 6-month datasets suffered from a substantial and selective number of non-responders (see Results), we did not analyse the repeated data structure but focused on the 12-month health outcomes. The main analyses of this trial consisted of a crude comparison between the mean change SF-36 scores from baseline to follow-up at 12 months. Between-group differences of the mean change scores were expressed in 95% CI. Additionally, we analysed the treatment effects on the 12-month SF-36 scores using multivariable linear regression, including the baseline SF-36 scores and the stratification variable (three types of functional symptoms) into the model. For the continuous secondary outcome, we used the same statistical approach. Between-group difference of the proportion of participants of whom the symptoms had greatly improved at follow-up and between-group difference of the proportion in care use were also expressed in 95% CIs. Baseline differences between participants who did or did not complete the interim follow-up assessments were analysed using a two-group Student’s t-test, χ2 test or Fisher’s exact test, when appropriate. p Values <0.05 were considered statistically significant. The p value for the ALDS was based on the original units of measurements (logits). All analyses were performed in IBM SPSS Statistics V.22.
Results
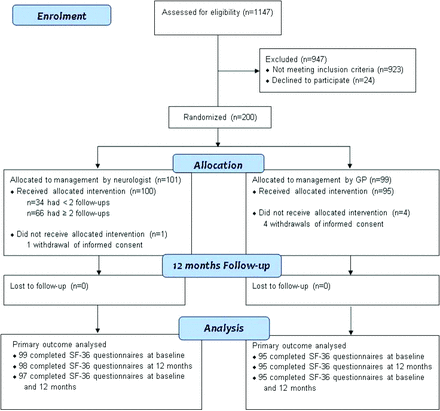
During the study period between August 2009 and November 2013, the diagnosis FNS was established in 1147 patients by 10 neurologists at our centre; each neurologist approximately screened 100 patients for inclusion and exclusion criteria. A total of 224 patients were eligible. The main reason for exclusion of 923 patients was the duration of the functional symptoms since the first consultation at the office of the GP of more than 1 year, whereas 24 declined to participate. Of the 200 randomised participants, 101 were allocated to initial management by the neurologist (intervention group) and 99 to immediate management by the GP (control group). Shortly after randomisation, five participants (one in the intervention and four in the control group) declined to fill out their baseline assessment forms and withdrew their informed consent. Therefore, the intervention and control arm consisted of 100 and 95 participants, respectively. None of them was lost to follow-up at 12 months.
After the explanation of the symptoms at the baseline visit, 34 participants of the intervention group did not adhere to the guideline of at least two follow-up visits: seven participants were of the opinion that follow-up visits were not necessary; this was also the case in 14 participants after their first follow-up visit: eight participants did not return after the baseline visit, whereas four participants did not return after the first follow-up visit. In one participant, the neurologist decided after the first follow-up visit that a second visit was pointless. A total of 27 participants had two follow-up visits, and 39 asked for more follow-up visits: three visits (n=20); four visits (n=10) and five or more visits (n=9) (see also figure 1). Duration of the follow-up visits ranged from 20 to 30 min.
Enrolment, randomisation and follow-up of patients. Participants were assessed for eligibility by neurologists to whom GP’s had referred. Participants were randomised by neurologists to whom neurologists who did the assessment had referred. GP, general practitioner; SF-36, 36-item Short Form Health Survey.
Table 1 shows the baseline characteristics of the included participants. The groups were generally well matched, with more absence from work in the intervention group (55% vs 45% control group) and a lower level of emotional role functioning (SF-36 subscale) in the control group (49.10, SD=44.93) compared with the intervention group (65.15, SD=44.68).
Baseline demographic and clinical characteristics. Figures are numbers (percentages*) unless stated otherwise
Despite reminder telephone calls of the research nurse at the 3 and 6 months interim follow-ups, 65 participants (65/195=33%) did not fill out and return their health questionnaires at 3 months, whereas 80 participants (80/195=41%) did not return the questionnaires at 6 months. Taken together, almost half of the participants (94/195=48%) were non-responders at the 3 and/or 6 months interim follow-ups. There were significant baseline differences between these 94 non-responders and the 101 participants who completed the interim follow-up assessments. Compared with the completers, non-responders had more frequently clusters of functional symptoms related to pain and ‘positive’ sensory symptoms (p=0.02), were younger (p=0.02), had more often a non-Caucasian ethnic background (p<0.001), were more frequently unfit for paid work (p=0.008) and tended to have more pain-catastrophising thoughts (p=0.08) and a lower general health perception on the SF-36 (p=0.07). No association was observed between type of allocation arm and number of non-responders (p=0.47).
Table 2 summarises the primary treatment outcome in terms of SF-36 scores. Participants in both treatment groups showed HRQL improvements on almost all SF-36 subscales. With the exception of the subscale ‘emotional role functioning’ and the Mental Component Summary score, no significant between-group differences in mean change scores were observed. Multivariable linear regression analysis (12-month follow-up scores as the dependent variable), adjusting for both the baseline scores and stratification variable, showed no significant treatment effect on the 12-month SF-36 outcome scores.
Primary treatment outcome: SF-36 subscale scores
Participants in both treatment groups showed health improvements on most secondary outcome scales (table 3). Within-group health changes over time were observed on the VAS pain (control group), the PCS, the somatisation SCL-90 subscale, the HADS and the VAS well-being. There were no significant between-group differences in mean change scores. Neither could we demonstrate treatment effects on these 12-month outcome parameters when using multivariable linear regression.
Secondary treatment outcomes
There was no significant difference between the treatment arms with regard to the number of participants who reported their symptoms at 12 months to have greatly improved compared with baseline: 29 participants (29/98=29.6%; two missing values) in the intervention group versus 31 participants (31/95=32.6%) in the control group (95% CI of the difference between proportions: from −16.1% to 10%). The distribution of the other scoring categories reflecting perceived health change over time (somewhat improved, remained the same, somewhat deteriorated and greatly deteriorated) was similar between the groups. (Data not presented, but available on request). We did not observe between-group differences in the use of physical therapy and psychotherapy during the last 6 months. Physical therapy: 38 participants (38/97=39.2%; three missing values) in the intervention group versus 45 participants (45/95=47.4%) in the control group (95% CI: from −22.2% to 6%). Psychotherapy: 23 participants (23/97=23.7%; three missing values) in the intervention group versus 27 participants (27/95=28.4%) in the control group (95% CI: from −17.1% to 7.7%).
Discussion
The results of this study in patients with FNS show that participants in both groups had improved on the primary and secondary outcome measures. About one-third of the participants reported their symptoms at 12 months to have greatly improved compared with baseline. All improvements were irrespective of type of treatment. This absence of a difference between the treatment arms was unexpected as we surmised that outcome would be better in patients who had at least two follow-up visits after the explanation of the diagnosis. This is because neurologists would have more authority than GPs in convincing these patients with neurological symptoms of the diagnosis. GPs with whom we discussed the results of this study were not surprised because they felt they can take over the management of these patients if the diagnosis has appropriately been explained by neurologists.
The absence of a difference between the treatment arms cannot be explained by the relative large number of participants (one-third) in the intervention group that did not adhere to the guideline of at least two follow-up visits. Of these 34 participants, 21 informed us to have had sufficient information and that they understood what the diagnosis was and what they had to do. This leaves only 13 participants that did not comply to the intervention protocol.
One of the methodological strengths of our study is the use of the modified informed consent procedure to mask the participants for the treatment options. Had we not used this procedure, we might have introduced ‘Hawthorne effects’, biasing the participants’ scores on the self-report outcome measures. Another strength is the number of included participants. In randomised studies in patients with FNS, only small numbers of participants could be included, except in the study of Sharpe et al 13 (n=127 patients), which was incorrectly excluded by Cochrane reviewers who used inappropriate inclusion criteria.14 Recently, Lehn et al 15 reviewed the studies on treatment in FNS; using this review, we calculated that in total 468 patients were included in 10 randomised trials. After this review, another trial16 was published, making the total number of included patients 528.
A major weakness of our study is that standard care was not the usual standard care in patients with FNS and was even not the local practice. The results therefore cannot be extrapolated to other practices. The diagnosis FNS was established by neurologists who briefly discussed the diagnosis and referred the participants to other neurologists with special interest in FNS who had scheduled half an hour for discussion on the diagnosis. In this study, 90% of the patients were randomised by one neurologist, which was far from ideal. We do not expect to have found the same results if the standard care had been given by the neurologists of this study who established the diagnosis. However, if we wish to study treatment effects in patients with FNS, we need to deviate from what is common practice. Not all neurologists are interested in FNS and of those who are, few apparently wish to randomise these patients. The number of randomised patients with FNS should be increased. At present, the average number is only 24 per group, which is far too low for sound conclusions. The model of stepwise inclusion we used may help in increasing the numbers.
Another weaknesses is the large number of patients that despite reminder telephone calls at interim follow-ups did not return their health questionnaires at 3 and/or 6 months. Although there were also clear baseline differences between the responders and non-responders, we could not incorporate available interim follow-up data in our analyses. Therefore, we had to focus solely on the change scores from baseline to the 12 months and were not able to get a more detailed picture of the health changes over time. The reason for this large number of non-responders was probably that many participants considered the questionnaires not relevant because they reported there was too much emphasis on psychological factors. In addition, they reported to be annoyed by the overlap of the type of measures in this study. Only with the help of the GPs could they be convinced to fill out the questionnaires at 12 months. Although our sample size calculation was based on 12-month outcomes, the statistical power of the study was not influenced by this large non-response rate. In future pragmatic trials, we advise to use short, less time-consuming outcome measures without overlap in content and with less emphasis on psychological aspects. In this study and that of Nielsen et al,16 the SF-36 physical domain was the most promising primary outcome, as this outcome correlated best with patients’ perceived change of symptoms.
In this study, only 20% of 1147 participants were eligible. The main reason for exclusion was the duration of symptoms. Moreover, many participants did not know exactly when the symptoms started and which symptom in the past was a symptom of FNS, and neurologists had difficulty with including persons who for instance were known to have fibromyalgia and developed symptoms of FNS. The number of included participants can be improved if participants who had no treatment for FNS irrespective of the duration are included.
There is few evidence on what the best management is in these patients. Can the information on the diagnosis be improved by better information on websites, and what should be the next management step after explanation of the diagnosis? During the last century, a large number of treatments have been recommended, but the publication of the first randomised controlled trial appeared not earlier than in 2002. We now have the results of 11 published randomised trials, but a meta-analysis of the data is not possible, as different subgroups of conversion disorder were included, different treatments tested and a variety of outcome measures were used. From these studies, we may conclude that physiotherapy and some form of cognitive behavioural therapy are promising. A study of Hubschmid et al 17showed beneficial effects of a brief psychotherapeutic intervention in collaboration with neurology consultants. However, assessment of outcome was not blind and recorded by one of the therapists.
In our next study, we wish to compare treatment consisting of explanation by trained neurologists, web information on FNS followed by physiotherapy and management by an informed GP versus the treatment regimen comparable with that of Hubschmid et al.17 We expect to include a sufficient number of patients by our stepwise inclusion model as in this study and inclusion irrespective of the duration of symptoms. We will use the SF-36 physical domain as primary outcome.
Acknowledgments
We thank Nadine Fleitour, research nurse, for the planning and implementation of the surveys, and collecting the outcome data. We thank the registrars and neurologists of the Department of Neurology of the AMC for the diagnosis and management of patients with conversion disorder.
References
Footnotes
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed
Presentation statement Marc Pleizier, registrar in neurology, died July 10, 2009, after having designed and organised this study. We dedicate this article to him.