Article Text
Abstract
Background Higher docosahexaenoic acid (DHA) intake is inversely correlated with relative risk of Alzheimer’s disease. The potential benefits of DHA supplementation in people with mild cognitive impairment (MCI) have not been fully examined.
Objective Our study aimed to assess the effect of a 24-month DHA supplementation on cognitive function and amyloid beta (Aβ)-mediated autophagy in elderly subjects with MCI.
Methods This was a randomised, double-blind, placebo-controlled trial in Tianjin, China. A total of 240 individuals with MCI were identified and randomly divided into intervention (DHA 2 g/day, n=120) and control (corn oil as placebo, n=120) groups. Cognitive function and blood Aβ-related biomarkers were measured at baseline, 6, 12, 18 and 24 months. Data were analysed using generalised estimating equation.
Results A total of 217 participants (DHA: 109, placebo: 108) completed the trial. During the follow-up, scores of full-scale IQ, verbal IQ and subdomains of information and digit span were significantly higher in the intervention group than the convention group (p<0.05). In the intervention group, blood Aβ-42 level and expression of Aβ protein precursor mRNA were decreased (p<0.05), while Beclin-1 and LC3-II levels and expression of LC3-II mRNA were increased (p<0.05).
Conclusion Daily oral DHA supplementation (2 g/day) for 24 months may improve cognitive function and change blood biomarker-related Aβ-mediated autophagy in people with MCI. Larger longer-term confirmatory studies are warranted.
Trial registration number ChiCTR-IOR-15006058.
- alzheimer’s disease
- cognition
- epidemiology
- neurobiology
- randomised trials
Statistics from Altmetric.com
Introduction
Alzheimer’s disease (AD) is the most prevalent, severe and disabling cause of dementia. So far, no pharmacological interventions have changed the onset or progression of AD. Mild cognitive impairment (MCI) is possibly the earliest stage of detectable dementia1 and may be the optimal time to intervene with preventive therapies.2 Modifying lifestyle habits to delay the onset of AD or MCI has attracted special attention, especially strategies that improve nutrition.3
Docosahexaenoic acid (DHA) (22:6 n-3) is the most abundant omega-3 polyunsaturated fatty acid (PUFA) in the brain, and it is tightly involved in the functioning of the central nervous system,4 particularly in neurogenesis, synaptogenesis and synaptic transmission.5 6 Consumption of omega-3 PUFA is associated with a decreased risk of AD in human observational studies and exerts protective effects on cognition and pathology in animal models.7–10 In recent years, DHA has gained much attention due to promising results that suggest it may be a potential tool to treat AD. However, results have been inconclusive.11 12 The mechanisms still remain unclear.
The deposition of amyloid plaques, a key hallmark of AD, is possibly one of the primary causes of neuronal loss in AD.13 Aberrant protein aggregation, including amyloid beta (Aβ) peptide accumulation, has been proved to play a key role in the initiation of AD.14 Therefore, a potential beneficial method for AD control is to attenuate Aβ depositions and protect neurons. Protein aggregates in the cell are cleared by autophagy, a mechanism impaired in AD.
Autophagy is a degradation pathway for the turnover of dysfunctional organelles or aggregated proteins in cells. A role for autophagy in Aβ metabolism has been suggested.15–17 Defective lysosomal proteolysis and compromised autophagosome transport occurs in AD, resulting in an accumulation of autophagosomes as well as Aβ peptides and Tau protein aggregates.18 Autophagosomes generate and contain Aβ,19 and oxidative stress-induced autophagy increases Aβ generation.20 In the battle against AD, autophagy stimulation is a promising therapeutic option—both as a new option and as a newly uncovered old strategy. DHA and molecules derived from them are known to have autophagy and pro-resolving properties, presenting a potential mechanism for these protective effects, but lack of population-based evidence.21 22
This study aimed to identify whether 24-month DHA supplementation would improve cognitive function by enhancing Aβ-mediated autophagy in Chinese elderly with MCI.
Methods
Sampling methods and ethical considerations
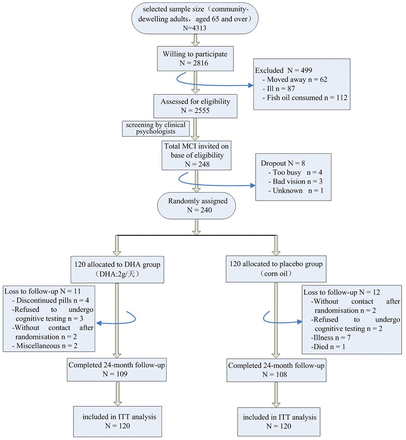
This was a randomised, double-blind, placebo-controlled trial that evaluated effects of 24-month DHA supplementation on cognitive function and Aβ-mediated autophagy in elderly subjects with MCI. Participants were enrolled between March 2013 and April 2013 according to published criteria23 as follows: (1) aged 65 years and over; (2) in generally good health, ambulatory and with sufficient hearing and vision for compliance with testing procedures; (3) absence of terminal illness or mental disorders (ie, major depression, schizophrenia, bipolar disorder, etc); (4) not using any nutritional supplementation known to interfere with nutrition status, fatty acid composition metabolism or cognitive function in the 3 months before recruitment; (5) not living in a nursing home or being on a waiting list for a nursing home. The diagnosis of MCI at baseline was determined according to the criteria of Petersen.24 Sociodemographic information and medical history were collected as previously described.23 The flow of study participants has been shown in figure 1.
Flow diagram for enrolment, randomisation and follow-up in the trial. DHA, docosahexaenoic acid; ITT, intention to treat; MCI, mild cognitive impairment.
Each subject provided written informed consent prior to study entry. This trial has been registered with trial number ChiCTR-IOR-15006058 (http://www.chictr.org.cn/showproj.aspx?proj=10530).
Assessment of cognitive function
As regards the assessment of cognitive performance, the subjects underwent a standardised neuropsychological assessment made by a senior psychologist at baseline, 6-month, 12-month, 18-month and 24-month time points during treatment. The main outcome in the current study was cognitive function, which was measured using the Chinese version of the Wechsler Adult Intelligence Scale-Revised (WAIS-RC).25 Cognitive domains were examined with the following most widely used tests: information, similarities, vocabulary, comprehension, arithmetic, digit span, block design, picture completion, digit symbol-coding, object assembly and picture arrangement. We used age-appropriate norms from the Chinese standardisation to calculate the IQ and index scores.25
Treatments procedures
The study consisted of a screening visit, followed 1 week later by a baseline visit, and four follow-up visits. After baseline screening, eligible participants were assigned randomly into the DHA group or the placebo group. The randomisation sequence was computer generated by the study sponsor.
The nutritional composition of the study drug was algal-derived DHA, administered as capsules, dosed as 2 g/day. The product was manufactured by Martek Biosciences, Columbia, Maryland. The DHA dose was selected based on evidence that plasma levels increase in a dose-dependent manner up to approximately 2 g/day, while at higher doses no further increase in plasma DHA is seen. The placebo was prepared by the manufacturer with identical capsules, using corn oil without DHA with the appropriate colourings and additives to give the placebo capsules the same consistence and colour. There is no evidence that corn oil intake at this amount has any benefits for cognitive function.
The supplements were packaged into identical pots, each containing 180 capsules, and labelled by staff who were not involved in the study. A blinding key linked each participant number to his or her assigned treatment. This key was kept by an investigator, not involved in any data collection or analyses, in a secure electronic file. The code was revealed at the completion of the trial following analyses of the main study aims. All project staff were unaware of group assignments until the completion of the trial and after data analysis.
Compliance with the trial protocol was assessed using self-reported number of days on which capsules were taken, a count of the number of capsules returned and plasma fatty acid composition measured from fasting venous blood samples collected from all participants willing to provide blood at baseline and at the 6-month, 12-month, 18-month and 24-month assessments for both groups.
Laboratory assay
Fasting venous blood samples were collected at baseline, 6-month, 12-month, 18-month and 24-month intervention. Blood samples, drawn in 2×5 mL serum-separating tubes, were posted overnight to a handling laboratory. Serum DHA concentrations were analysed by means of gas liquid chromatography using flame ionisation detection on a Schimadzu G-2010 (Schimadzu, Sydney, Australia). DHA concentrations were expressed in absolute concentrations (μmol/L) unless otherwise stated. Gene expression for Aβ protein precursor (APP) mRNA, Beclin-1 mRNA, P62 mRNA and LC3-II mRNA was quantified by real-time PCR. The assay was performed using the Roche LightCycler 480 sequence detector (Roche, Mannheim, Germany). Protein levels of Aβ-40, Aβ-42, APP, β-secretase 1 (BACE1), Beclin-1, LC3-II and P62 were assessed by western blot assay. Proteins were detected by chemiluminescence assay and then quantified by densitometric analysis using NIH ImageJ software (V.1.61). The intensity of each protein band was normalised to the respective β-actin band. Laboratory measures were conducted on coded samples by workers blinded to other data, including intervention group.
Statistical analysis
Baseline characteristics of study population have been checked by means of χ2 tests or Fisher’s exact test for categorical variables and one-way ANOVA for continuous variables, with post hoc comparison using the Bonferroni test for multiple comparisons. A generalised estimating equation (GEE) with an exchangeable working correlation matrix was used to estimate a combined effect for the difference between treatments. The results are presented as estimates (with 95% CI) of the difference between the two treatments, for both time periods combined, for each test of cognition. The difference between the treatments for each variable was estimated after adjusting for its baseline values in the first model and its baseline values, sex and education in the second model. All analyses were performed with the intent-to-treat principle. A two-sided p value of 0.05 or less was considered to be statistically significant. All calculations were made using SPSS software package V.16.0 (SPSS).
Results
Participant flow, characteristics and follow-up
At baseline, treatment groups were similar for demographic, health, fish consumption and cognitive function. Baseline characteristics of the study population are shown in table 1. Drop-out rates over the 24 months after randomisation were similar between the two groups: 11 (9.17%) participants dropped out from the DHA group and 12 (10.00%) from the placebo group (χ 2 20.294, p=0.778).
Baseline characteristics of the study population
Changes of serum DHA concentration
The mean serum DHA levels (μmol/L) in the DHA group were 286 (95% CI 269 to 304) at baseline, 291 (95% CI 267 to 308) at 6th month, 295 (95% CI 271 to 319) at 12th month, 296 (95% CI 269 to 322) at 18th month and 299 (95% CI 275 to 324) at 24th month, as compared with 285 (95% CI 268 to 297) at baseline, 284 (95% CI 266 to 299) at 6th month and 287 (95% CI 263 to 295) at 12th month, 286 (95% CI 274 to 299) at 18th month and 287 (95% CI 274 to 302) at 24th month in the placebo group. The mean serum DHA levels showed substantial percentage increases in both groups (difference: −0.87; 95% CI −1.93 to −0.18; p=0.022) and was greater in the intervention group (+4.55%) compared with the control group (+0.35%).
Cognitive status
GEE revealed few significant interaction effects over the 24-month period for all of the neuropsychological tests except full-scale IQ (FSIQ), verbal IQ (VIQ), information and digit span tests. Regarding the FSIQ, the mean score in the intervention group was significantly higher than in the control group (difference: –1.13; 95% CI −2.22 to −0.02; p=0.037), and the difference remains significant after adjustment (difference: −0.79; 95% CI −1.88 to −0.11; p=0.018). The mean score on the VIQ was higher in the intervention group than in the control group (difference: –1.21; 95% CI −2.31 to −0.11; p=0.019), and the difference still remains significant after adjustment (difference: −0.92; 95% CI −1.98 to −0.22; p=0.027). Based on the analysis of each domain, the mean score on information tests was higher in the intervention group than in the control group (difference: –1.29; 95% CI −2.38 to −0.13; p=0.038). Further adjustment did not alter the difference (difference: –0.89; 95% CI −1.97 to −0.22; p=0.023). On digit span tests, the mean score was higher in the intervention group than in the control group (difference: −1.08; 95% CI −1.14 to −1.04; p=0.007), and the difference remains significant after adjustment (difference: −1.17; 95% CI −1.23 to −1.12; p=0.008). All the other tests did not show significant difference (p>0.05) (table 2).
Differences in cognitive domain scores between the two groups during the study period
Blood of Aβ-related biomarkers
GEE showed that over 24 months, regarding the blood biomarkers of Aβ-mediated autophagy, the Aβ-42 level was significantly lower in the intervention group than in the control group before (difference: –1.07; 95% CI −1.14 to −1.02; p=0.024) and after adjustment (difference: –1.14; 95% CI −1.21 to −1.08; p=0.032), as was the APP mRNA level (before adjustment, difference: −1.48; 95% CI −3.12 to −0.66; p=0.038; after adjustment, difference: −1.15; 95% CI −3.08 to −1.27; p=0.027). However, no significant differences in Aβ-40, APP and BACE1 were observed (table 3).
Differences in blood Aβ-related biomarkers between the two groups during the study period
Blood autophagy biomarkers
Regarding the blood biomarkers of autophagy, these findings suggest that Beclin-1 expression is higher in the intervention group than in the control group (difference: –0.18; 95% CI −0.30 to −0.09; p=0.035). Further adjustment did not alter the difference (difference: –0.25; 95% CI −0.31 to −0.19; p=0.027). The LC3-II level was lower in the intervention group than in the control group (difference: −1.28; 95% CI −2.10 to −0. 47; p=0.011), and the difference remains significant after adjustment (difference: −1.30; 95% CI −2.28 to −0.33; p=0.007). The LC3-II mRNA expression was higher in the intervention group than in the control group (difference: −0.12; 95% CI −0.19 to −0. 04; p=0.027), and the difference remains significant after adjustment (difference: −0.16; 95% CI −0.24 to −0.08; p=0.015). However, no significant differences in P62, Beclin-1 mRNA and P62 mRNA were observed (table 4).
Differences in blood Aβ-related biomarkers between the two groups during the study period
Discussion
In this randomised controlled trial (RCT), daily oral DHA supplementation(2 g/day)for 24 months significantly improved cognitive function in tests of global cognitive function (FSIQ in WAIS-RC), VIQ, information and digit span tests. In addition, we observed a significant decrease in peripheral blood Aβ-42 concentrations and APP mRNA expression and increase in Beclin-1, LC3-II and LC3-II mRNA expression, compared with the placebo group (p<0.05). These findings support the hypothesis that the neuroprotective role of DHA may associate with enhancing Aβ-mediated autophagy.
Poor performance on memory tests is a main characteristic of the cognitive deficits in patients with MCI. Considering the phenomenology, memory can be classified by its duration: short-term memory, which lasts minutes to 1–3 hours, and long-term memory, which lasts hours to days or years.26 In this trial, DHA supplementation beneficially affected global cognitive function, specifically participants’ performance on the information and digit span tasks. Information test is a valid indicator of long-term memory, while digit span test examines attention/short-term memory.27 The direct role of the DHA modulation in specific regions of the brain is likely an important contributor to memory development and continued normal memory function.28 In AD, DHA seems to be markedly lower in the hippocampus, a brain region intimately involved in memory formation and retrieval.11 It remains to be determined whether this relatively specific change in brain DHA is in part a function of inadequate DHA intake or specific pathological mechanisms more rapidly destroying DHA in the hippocampus than elsewhere in the brain.
Extracellular accumulation of Aβ peptide has been reported to be a major cause of AD, and large numbers of autophagic vacuoles accumulate in the brain of patient with AD.29 30 DHA-induced autophagy was monitored by measuring the mRNA and protein levels of Beclin-1, LC3-II and P62.31–33 In this trial, daily use of DHA supplementation has significantly reduced blood Aβ-42 concentrations and APP mRNA expression and increased blood Beclin-1, LC3-II concentrations and LC3-II mRNA expression in elderly individuals with MCI. Our findings suggest a comprehensive protection of DHA against cognitive decline, decreasing Aβ production and aggregation in the brain and increasing its clearance from the brain.34 These highlight the importance of DHA from a pharmacological perspective.
MCI is associated with an increased risk for progression to AD (10%–15% per year), which is 10 times that in normal population. At each follow-up, we have already given a clinical diagnosis of AD based on National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria.35 On the basis of the data collected in 2 years, incidence density of MCI conversion to AD in the intervention group was 9.70% (9.52%, 10.29%), while that in the control group was 9.75% (9.56%, 9.67%). By log-rank test, there was no significant difference on the outcome of cognitive decline between the two groups (χ2=0.643, p>0.05). That means conversion rate of MCI progressed to AD in intervention group is similar to that in control. Because individuals with MCI decline at different rates and some never develop AD, the conversion rate can keep steady after 5–6 years. Thus, we decided to increase intervention time and cost of the trial. Over a follow-up period of approximately 5–6 years, we will examine the conversion of MCI to AD and assess both the rates over time and factors associated with conversion.
Our study offers several advantages arising from its rigorous design. First, the random assignment of MCI subjects resulted in a strong resemblance of the two groups with respect to most background variables so that comparisons between both groups primarily reflect the effect of DHA. Second, we used a standard measure of cognitive function. WAIS-RC has good reliability, internal consistency and validity. Third, MCI and probable dementia were identified using rigorous, expert adjudicated diagnoses. Finally, loss to follow-up over 24 months was <15%.
Several limitations have to be noted. First, the optimal dosage of DHA needed to improve cognitive function is unknown. Second, the current study used methods that determined the blood concentrations of DHA, which might not reveal DHA status in tissues. Third, compared with other results, blood DHA concentrations in our study may be slightly higher, which may be associated with our research site. The trial was conducted in Tianjin city, China, close to the Bohai sea. The sea off Tianjin abounds with quality seafood. Selection bias is unavoidable. Rigorous RCT design can better reduce selection bias and other confounding factors.
In summary, we found obvious evidence that daily oral DHA supplementation (2 g/day) for 24 months can significantly improve cognitive function in subjects with MCI. The beneficial effect of DHA supplementation on cognitive function may act through an Aβ-mediated autophagy mechanism. Larger-scale double-blind placebo-controlled randomised trials of longer duration are needed.
Acknowledgments
The authors thank all of the subjects for their participation.
References
Footnotes
Contributors FM: study concept or design and critical revision of the manuscript; Y-PZ: analysed data or performed statistical analysis and drafting of the manuscript for content; YL and JH: field survey and data collection; RM: hands-on conduct of the experiments and data collection. No potential conflicts of interest relevant to this article were reported.
Funding This study was supported by grants from 2012 Chinese Nutrition Society Nutrition Research Foundation—DSM Research Fund (grant number: 2014-003) and the National Natural Science Foundation of China (grant number: 81573148).
Competing interests None declared.
Patient consent Obtained.
Ethics approval This study adheres to the principles of the Declaration of Helsinki. The protocol was approved by the Ethics Committee at Tianjin Medical University, China.
Provenance and peer review Not commissioned; externally peer reviewed.